Dr. Pablo Sepúlveda 1 , Dra. Alberto Laporte 1 , Dra. Andrea Fuentealba 1 , Dr. Luis Ignacio Cortínez 2
Rev Chil Anest Vol. 40 Núm. 2 pp. 122-137|doi:
PDF|ePub|RIS
Introducción
The recently published pharmacokinetic (PK) propofol model designed to be used in TCI mode in normal and obese patients1 has not included an effect site model to predict the time course of the effect. The goal of this study is derive a PD model extracted from a morbidity obese patient’s population.
Objetivo General
The goal of this study is derive a PD model extracted from a morbidity obese patient’s population.
Material y Métodos
With Ethic Institutional Committee approval, patients proposed for bariatric surgery, using standard monitoring, arterial line and BIS®, received a plasma TCI with the study model using Anestfusor®. After the patients reach a calculated target of 12 µg/ml we set the target in 0 until patients wake up (BIS > 75) obtaining a complete BIS depression-recovery curve and the correspondent calculated Cp. With Anestfusor® the BIS and Cp calc data where stored every 5 sec. Using NONMEM a complete IMAX model was derived and the plasma effect-site elimination rate constant (ke0) was used to link PK model predictions with BIS response data. A performance analysis compa-ring the measured and the obtained predicted BIS values for each calculated effect site concentration was done. No other drugs were given. Arterial blood samples for propofol assays were collected at 1,2,3,5,9 and 10 min to evaluate the PK performance of the model using Varvel2 methodology. The sample where analyzed in HPLC.
Resultados
Eleven ASA I-II obese patients (47,7 ± 11 years, BMI 44 ± 6 kg/m2, weight 122 ± 19 kg, height 169 ± 9,9 cm) were studied. With basal BIS of 94, the PD model obtained a ke0 of 0,21 min-1, gamma 3,67 and an EC50 3.29 µg/ml. The performance error from the measured BIS vs predicted BIS with the PD model was MDPE 0,55% (percentile 25-75: -19-14) and MADPE 15,8% (percentile 25-75:5-32). Pharmacodynamic time profiles of the measured observations vs predicted BIS for the obtained model are shown in Figure 1. The FK performance error during the studied time was MDPE -7% and MAPE 19%.
Conclusiones
The time profile of propofol BIS effect in obese patient was well described (MDPE between ± 20 and MDAPE < 30%) with the extracted PD parameters (ke0 and Ce50) and allowed a good characterization of propofol TCI effect site mode in this population.
Referencias
-
Cortínez LI, Anderson BJ, Penna A, et al. Influence of obesity on propofol pharmacokinetics: derivation of a pharmacokinetic model. Br J Anaesth 2010; 105: 448-56.
-
Varvel JR, Donoho DL, Shafer SL. Measuring the predictive performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm, 1992; 20: 63-94.
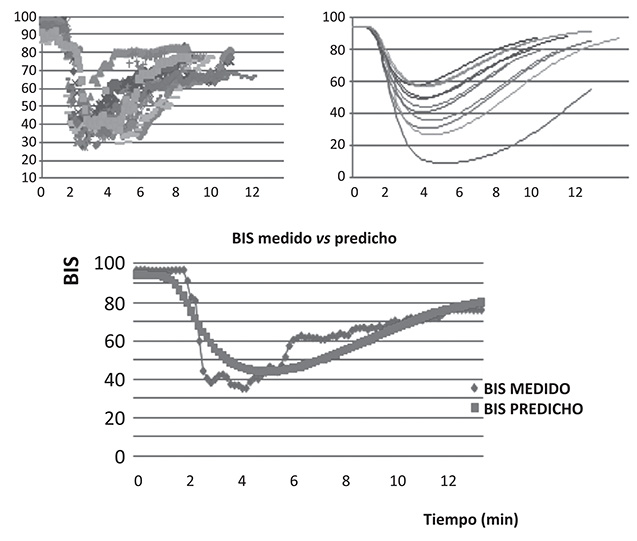
Figure 1


 Creative Commons Attribution
Creative Commons Attribution