Enrique Sepúlveda 1 ,*, Salvador Romero 1 , Reina Abujeta 2 , Estela Pérez 2
Recibido: 12-10-2021
Aceptado: 21-12-2021
©2022 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 51 Núm. 2 pp. 231-233|https://doi.org/10.25237/revchilanestv5110021207
PDF|ePub|RIS
Vía aérea difícil no anticipada por quiste subglótico: caso clínico
Abstract
Acquired subglottic cysts are an unusual cause of stridor. They are usually developed following a tracheal intubation episode in a premature patient. We report the clinical case of an ex-premature patient who was diagnosed as having an acquired subglottic cyst after causing a difficult intubation situation. This situation can potentially uncover the obstruction and initiate the complete diagnostic and therapeutic process.
Resumen
Una causa poco frecuente de estridor laríngeo corresponde a quistes subglóticos. En general se desarrollan luego de una intubación en un paciente prematuro. Se presenta el caso clínico de un ex prematuro que presenta una intubación difícil dada por la presencia de un quiste subglótico. Gracias a esta situación, se inició el estudio y tratamiento del quiste.
-
Introduction
-
Clinical case
Subglottic cyst was first described by Wigger and Tang in 1968 in a fatal subglottic obstruction case[1]. This lesion presents as risk factors the prematurity and prolonged in- tubation, although it has also been described in full term birth and in patients intubated for as low as 24 hours. Subglottic cyst presents an incidence of 0,05% of patients admitted in Neonatal Intensive Care Units, and an incidence of 0,69% in 24-27 weeks of gestational ag[2].
We present here a case about an acquired subglottic cyst that was diagnosed and treated after causing a difficult intubation situation.
An ex-premature infant of 5 months gestationally corrected age (GCA), 4.200 grams, 56 centimeters, was scheduled for vesicostomy closure surgery.
He was birth on a caesarean delivery on week number 27+2 of gestation due to intrapartum fetal distress. He was intubated at the second minute after delivery because of apnea and bradycardia and spent 98 days in Neonatal Intensive Care Unit, of which the 30 first days he stayed intubated. He suffered from bronchopulmonary dysplasia and received treatment with montelukast and aerosolized budesonide and salbutamol. He was operated before for vesicostomy placement, inguinal herniotomy and laser photocoagulation of chorioretinal adhesions.
On the day of surgery his mother explained the anesthetist that sometimes she could hear an estrange breathing noise from the baby, for the previous 3 months, without any breath- ing distress. On inspection, no breathing sound could be heard, pulse oximetry was 97% on room air, and chest auscultation was normal.
In the operation theatre the standard monitorization was applied and inhalational induction of anesthesia was accomplished with 6% sevoflurane and 80% oxygen. After peripheral vein cannulation, saline with 1% glucose was started at 20 ml/h and fentanyl 6 mcg, rocuronium 1,5 mg and dexamethasone 0,5 mg were administered.
Direct laryngoscopy showed a grade 1 Cormack-Lehane view and an orotracheal 4 mm inner diameter uncuffed endotracheal tube (ETT) was placed through the vocal cords, but it could not be advanced further into the trachea. Again, a 3,5 mm uncuffed ETT and later a 3 mm uncuffed ETT could not be advanced, and finally a 2,5 mm uncuffed ETT (3,6 mm outer diameter) could be advanced into the trachea. Intermittent face mask ventilation was performed between the intubation attempts, without any ventilation trouble.
Anesthesia was maintained with 2,4% sevoflurane, 0,1 mcg/kg/min remifentanil and 50% inspired oxygen. The surgery lasted 160 minutes and was performed without any in- convenience. After dexketoprofen 4 mg, paracetamol 60 mg and a caudal epidural block with 3 ml levobupivacaine 0,25%, sevoflurane and remifentanil were discontinued. The patient recovered spontaneous ventilation, was extubated, and was moved to Pediatric Intensive Care Unit (PICU) for vigilance.
After 12 h postoperatory he presented a biphasic stridor with desaturation up to 90% and was satisfactorily treated with oxygen supplementation through nasal cannula, addition- al intravenous dexamethasone and aerosolized budesonide and salbutamol.
After 72 h postoperatory vigilance he was discharged from PICU to the ward. He was scheduled for a diagnostic flexible bronchoscopy which allowed the diagnosis of an acquired sub-glottic tracheal cyst that occluded 60% of the tracheal lumen (Figures 1 and 2). Some days later he received laser endoscopic surgery in a reference center, where a subglottic cyst marsupialization was performed.
Subglottic stenosis and subglottic cyst share common risk factors and etiopathogenic mechanism. It is thought that sub-glottic cyst development is due to an ischemic lesion on the tracheal mucosa caused by intubation, and which is followed by a cicatrization process that would obstruct mucosal gland con- ducts, creating retention cysts. Most frequent symptoms are biphasic stridor, respiratory insufficiency and feeding difficulty, and these usually occur in the first 12 months following the intubation episode. Its therapeutic management implies securing the airway in case of an important airway obstruction. Surgical correction is usually performed as microsurgery or laser surgery, but its high recurrence rate of 43% makes medical follow-up necessary in the next months[2],[3],[4].
No difficult airway was suspected in our patient according to difficult airway predictors[5].
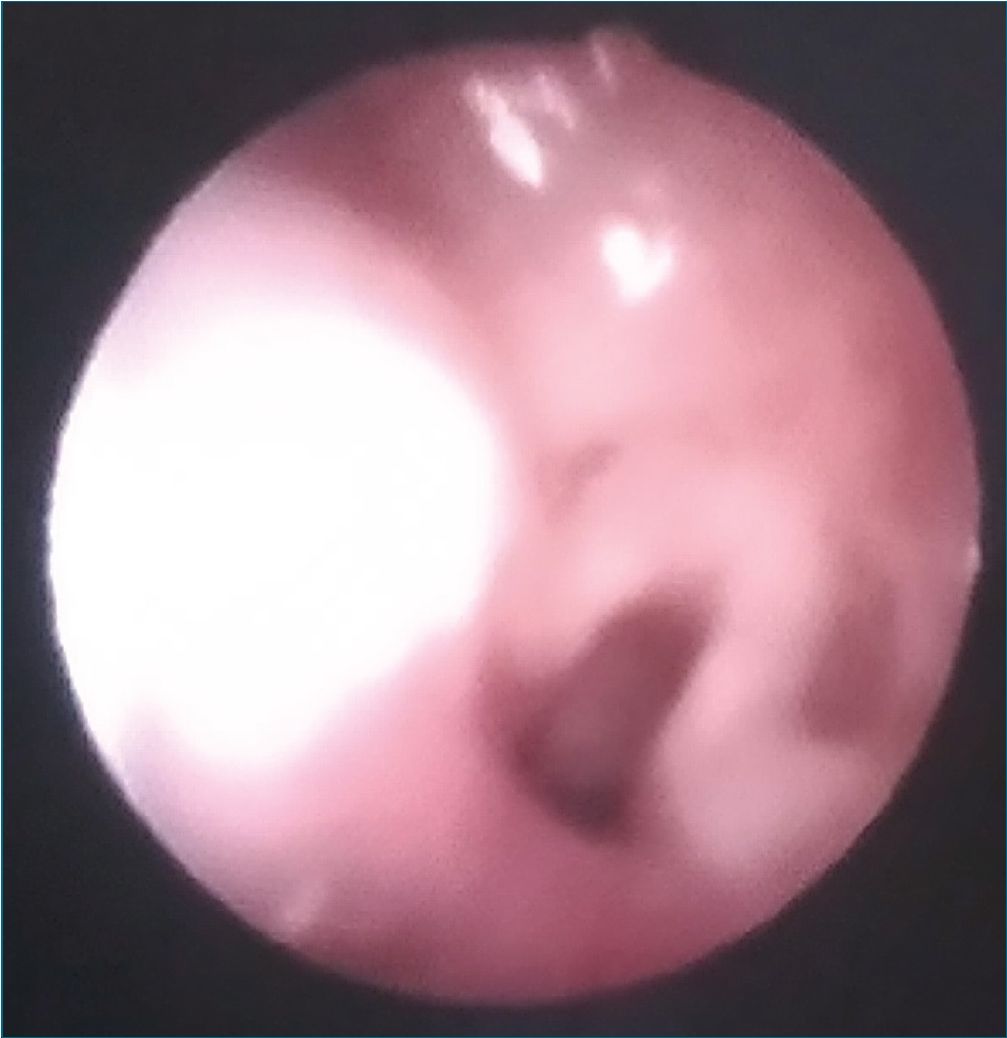
Figure 1. Original. Bronchoscopy view. Vocal cords and tracheal lumen can be identified.
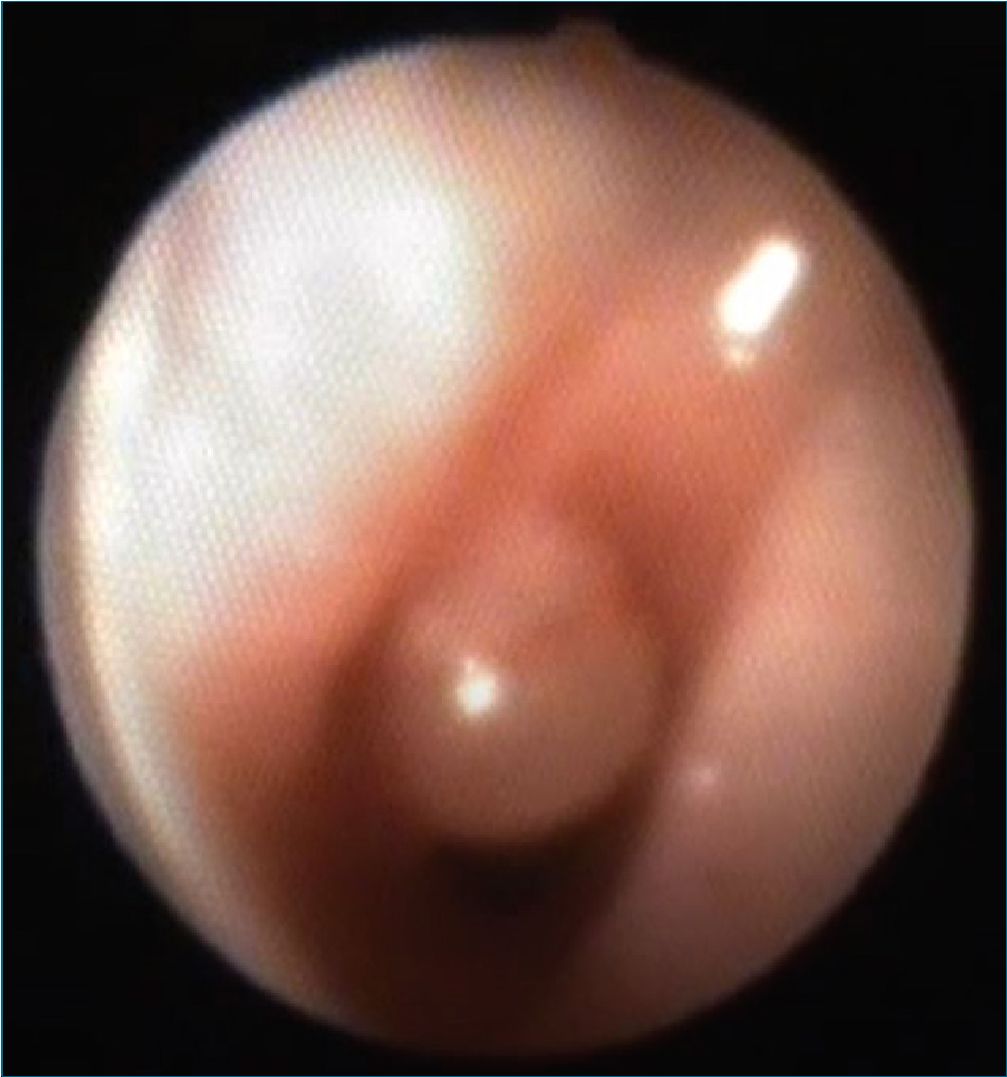
Figure 2. Original. Bronchoscopy view. The subglottic tracheal cyst can be identified on the left lateral wall of the trachea, protruding to the traqueal lumen.
Probably, the subglottic cyst that did not cause any symptom on a basal situation, could produce some tracheal obstruction with its typical biphasic stridor during upper airway infection episodes, as the mother referred some kind of breathing noise.
Cotton-Myer subglottic stenosis classification determines the percentage of tracheal stenosis that the patient presents when comparing the ETT size used in each situation with the ETT size that would theoretically fit a patient with the same age and with a normal airway[6].
The patient was intubated before in two occasions on his 1st month of age according to GCA, and medical records showed a 2,5 mm inner diameter ETT was used both times without any problem. This situation could be appreciated by the anesthesiologist as a mild tracheal lumen reduction, which would explain the absence of report regarding any an- esthetic difficulty on those surgeries. Moreover, on that age the patient had not presented any stridor, so another possibility is that the subglottic cyst had not developed yet at those times.
According to the GCA of our patient, the approximate ETT size was 3,5-4 cm inner diameter[7]. Whenever a difficult intubation situation presents, the main fact we have to consider is the possibility of ventilation and oxygenation, which was found easy in that situation. In our situation, after 3 attempts of intubation and with an easy ventilation, we suspected a reduced size of the subglottic lumen and decided trying again with a thinner ETT, which finally allowed intubation.
Our patient was intubated with a 2,5 mm inner diameter uncuffed ETT, which represents a grade 2 subglottic stenosis according to Cotton-Myer classification, meaning 51-70% stenosis of the normal tracheal size[6].
Subglottic tracheal cyst is a rare cause of stridor, and most patients had a previous prematurity and prolonged intubation. This lesion could cause a difficult intubation situation and post-
operatory vigilance in an Intensive Care Unit is recommended. This situation should also initiate the diagnostic and therapeutic assessment.
References
1. Wigger HJ, Tang P. Fatal laryngeal obstruction by iatrogenic subglottic cyst. J Pediatr. 1968 Jun;72(6):815–20. https://doi.org/10.1016/S0022-3476(68)80434-2 PMID:5652608
2. Agada FO, Bell J, Knight L. Subglottic cysts in children: a 10-year review. Int J Pediatr Otorhinolaryngol. 2006 Aug;70(8):1485–8. https://doi.org/10.1016/j.ijporl.2006.03.010 PMID:16650484
3. Wei JL, Bond J. Management and prevention of endotracheal intubation injury in neonates. Curr Opin Otolaryngol Head Neck Surg. 2011 Dec;19(6):474–7. https://doi.org/10.1097/MOO.0b013e32834c7b5c PMID:21986802
4. Lim J, Hellier W, Harcourt J, Leighton S, Albert D. Subglottic cysts: the Great Ormond Street experience. Int J Pediatr Otorhinolaryngol. 2003 May;67(5):461–5. https://doi.org/10.1016/S0165-5876(02)00406-8 PMID:12697347
5. Hsu G, Fiadjoe JE. The pediatric difficult airway: updates and innovations. Anesthesiol Clin. 2020 Sep;38(3):459–75. https://doi.org/10.1016/j.anclin.2020.05.001 PMID:32792177
6. Myer CM 3rd, O’Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. 1994 Apr;103(4 Pt 1):319–23. https://doi.org/10.1177/000348949410300410 PMID:8154776
7. Brambrink AM, Braun U. Airway management in infants and children. Baillieres Best Pract Res Clin Anaesthesiol. 2005 Dec;19(4):675–97. https://doi.org/10.1016/j.bpa.2005.07.002 PMID:16408541

 ORCID
ORCID