Victor Tortorici PhD. 1,2*, Cristina Maestres BSc.1, Nicole d’Escriván BSc.1, Rafael Martínez-Lombao MD.1, Richard C. Gershon PhD.3, and Marco Echeverria-Villalobos MD, PhD.4
Recibido: 13-01-2024
Aceptado: 23-01-2024
©2024 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 53 Núm. 2 pp. 177-183|https://doi.org/10.25237/revchilanestv53n2-122
PDF|ePub|RIS
Modulación cognitiva del dolor: Interacción entre atención, control inhibitorio y percepción del dolor
Abstract
Introduction: A reciprocal modulation between cognitive processing and pain experience has been hypothesized, suggesting overlapping regulatory interactions between the circuits involved that may represent a complementary route to traditional pain management. Objective: To determine whether a cognitive test involving attention and inhibitory control (Flanker Inhibitory Control and Attention Test, FICAT) can modify pain perception and cognitive performance in healthy subjects. Materials and Methods: 40 students from the Metropolitan University (UNIMET), aged 18-25 years, were subjected to three experimental conditions. First, participants underwent the Cold Pressor Test (CPT). Then, they performed the FICAT and finally performed the FICAT while simultaneously exposed to the CPT. Pain intensity was evaluated using the Visual Analog Scale (VAS), and tolerance was measured in seconds, reflecting the total exposure time to the painful stimulus. Results: Compared to the pain-free scenario, the simultaneous application of noxious stimulation and cognitive testing significantly improved the subjects’ tolerance to painful stimuli but did not reduce the intensity of pain perception. In addition, the combination of FICAT+CPT significantly improved executive performance, reflected in faster reaction times. Discussion: Our results suggest that exposure to a painful stimulus does not necessarily disrupt the level of attention and the ability to exert inhibitory control. Moreover, executive capacity appears to be enhanced by the acute painful situation. Our findings also indicate that a cognitive task increased tolerance among the participants. Conclusion: These results suggest that complementary non-pharmacological strategies may help to reduce the cognitive burden of pain and partially improve how pain is behaviorally tolerated.
Resumen
Introducción: Se ha planteado la hipótesis de una modulación recíproca entre el procesamiento cognitivo y la experiencia del dolor, lo que sugiere interacciones reguladoras solapadas entre los circuitos implicados, que pueden representar una vía complementaria al tratamiento tradicional del dolor. Objetivo: Determinar si una prueba cognitiva de atención y control inhibitorio (Prueba de atención y control inhibitorio de Flanker (Flanker Inhibitory Control and Attention Test, FICAT) puede modificar la percepción del dolor y el rendimiento cognitivo en sujetos sanos. Materiales y Métodos: 40 estudiantes de la Universidad Metropolitana (UNIMET), con edades comprendidas entre 18 y 25 años, fueron sometidos a tres condiciones experimentales. En primer lugar, los participantes se sometieron a la Prueba de Presión Fría (Cold Pressor Test, CPT). A continuación, realizaron la FICAT y, por último, realizaron la FICAT mientras se exponían simultáneamente a la CPT. La intensidad del dolor se evaluó mediante la Escala Visual Analógica (Visual Analog Scale, VAS), y la tolerancia se midió en segundos, reflejando el tiempo total de exposición al estímulo doloroso. Resultados: En comparación con el escenario sin dolor, la aplicación simultánea de la estimulación nociva y la prueba cognitiva mejoró significativamente la tolerancia de los sujetos al estímulo doloroso, pero no redujo la intensidad de la percepción del dolor. Además, la combinación de FICAT+CPT mejoró significativamente el rendimiento ejecutivo, reflejado en tiempos de reacción más rápidos. Discusión: Nuestros resultados sugieren que la exposición a un estímulo doloroso no altera necesariamente el nivel de atención y la capacidad de ejercer un control inhibitorio. Más aún, en nuestro caso, la capacidad ejecutiva pareció verse reforzada por la situación dolorosa aguda. Nuestros hallazgos también indican que una tarea cognitiva fue capaz de aumentar la tolerancia en los participantes. Conclusiones: Estos resultados sugieren que el uso de estrategias complementarias no farmacológicas puede ayudar a reducir la carga cognitiva impuesta por el dolor y mejorar, al menos parcialmente, la tolerancia al mismo.
-
Introduction
Pain is recognized as a multidimensional phenomenon linked and influenced by cognitive functioning[1]-[3]. Neuroimaging has demonstrated the anatomical and functional convergence of the pain neuromatrix with cognitively related neural substrates spanning attention and inhibitory control[4],[5], suggesting that pain and cognitive functions can exert reciprocal modulatory effects. Intense pain affects the performance of cognitive tasks, and cognitive strategies may promote distraction from concurrent pain[6]-[9]. However, the exact modulatory mechanism remains to be elucidated. The main goal of this study was to determine whether a cognitive test involving inhibitory control and attention could modify the perception of acute cold pain and reduce its cognitive burden in healthy subjects.
-
Materials and Methods
-
Sample
A total of 40 subjects (equal gender ratio), undergraduate students from UNIMET, volunteered in our laboratory and qualified for the study. Before starting the experiment, participants completed a survey to verify their health status. Based on this, 13 subjects were excluded because their answers matched at least one of the following conditions: current analgesic consumption, fatigue, unstable blood pressure, history of fainting or seizures, active/current injury, presence of physical pain, pregnancy, or current menstrual cycle.
-
Variables and experimental procedures
The variables measured in our experimental model were attention, inhibitory control, pain tolerance, and pain intensity. The application of FICAT helped to determine the first two. The test version, in Spanish, was administered on a 6th-generation iPad (Model A1893, Apple Computer, Inc., CA, USA). FICAT requires the subject to focus on a given stimulus (one horizontal arrow in the middle of the screen) while inhibiting attention to stimuli (several horizontal arrows), flanking it on both sides. At times, the middle stimulus pointed in the same direction as the “flankers” (congruent stimulus), and at other times appeared in the opposite direction (incongruent stimulus). The word “middle” appeared on the screen for all the participants as a reminder of where to focus. All test instructions were provided on the iPad screen. Before beginning the test, the examiner read the instructions to each participant and pointed out relevant aspects of the stimuli on the screen. A block of 20 trials per test was conducted for each participant. The test took approximately three minutes, and the NIH Toolbox automatically calculated the final score. There was a maximum of 10 points, five of which corresponded to the number of correct answers (computed accuracy), while the other five represented the reaction time for each item[10]-[13].
CPT has been widely adopted as an experimental model for acute nociceptive pain[20]. It consisted of immersing the participant’s non-dominant hand in a circulating water bath (Haake A81, ALT, CT, USA) at a controlled temperature (4°C) until the feeling became unbearable for the participant. Tolerance to acute pain was measured in seconds. The cutoff time for this test was preset at two minutes to avoid unnecessary discomfort. Participants could withdraw their immersed hands at any stage of the test. Pain intensity was recorded with the Visual Analogue Scale (VAS), consisting of a 100 mm strip that goes from 0, “no pain,” to 10, “worst pain imaginable.” VAS and nondominant hand temperature were measured (561 HVAC Infrared & Contact Thermometer, Fluke Corporation, WA, USA) before and after each experimental challenge (data not shown) to verify the absence of pain and the recovery of the skin temperature to baseline values before starting each experiment.
The participants were sequentially exposed to three experimental situations: the application of CPT alone (noxious stimulation), the administration of FICAT (cognitive test) without any additional stimulation, and the simultaneous application of FI- CAT and CPT to study their possible reciprocal interaction.
-
Statistical analyses
Data were analyzed using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). Normality was assessed using the D’Agostino-Pearson and Shapiro-Wilk normality tests. Regarding the sample size, nonparametric statistics were used in the subsequent analyses. The Mann-Whitney U-test was used to establish possible differences between the experimental conditions. The significance level was set at p < 0.05 and verified using 95% confidence intervals. The relationship between cognitive performance and acute pain was also discerned using the Spearman correlation coefficient, considering significant correlations starting at 0.30[15].
-
Standard protocol approval, registration, and participant consent
This study was approved by the Research Advisory Commission, a division of the Research and Development Directorate of UNIMET, before starting the experimental procedures, following the recommendations of the Declaration of Helsinki of the World Medical Association’s (WMA)[16]. The study also followed the Ethical Guidelines for Pain Research in Humans of the International Association for the Study of Pain[17]. Subjects were informed about the tasks they were about to perform and their signed consent to the investigation was requested before starting the study. Participants also signed a confidentiality agreement and gave authorization to use the information obtained from the study for academic and research purposes, including the publication of the findings. Finally, the identities of the participants were kept anonymous during each phase of this research.
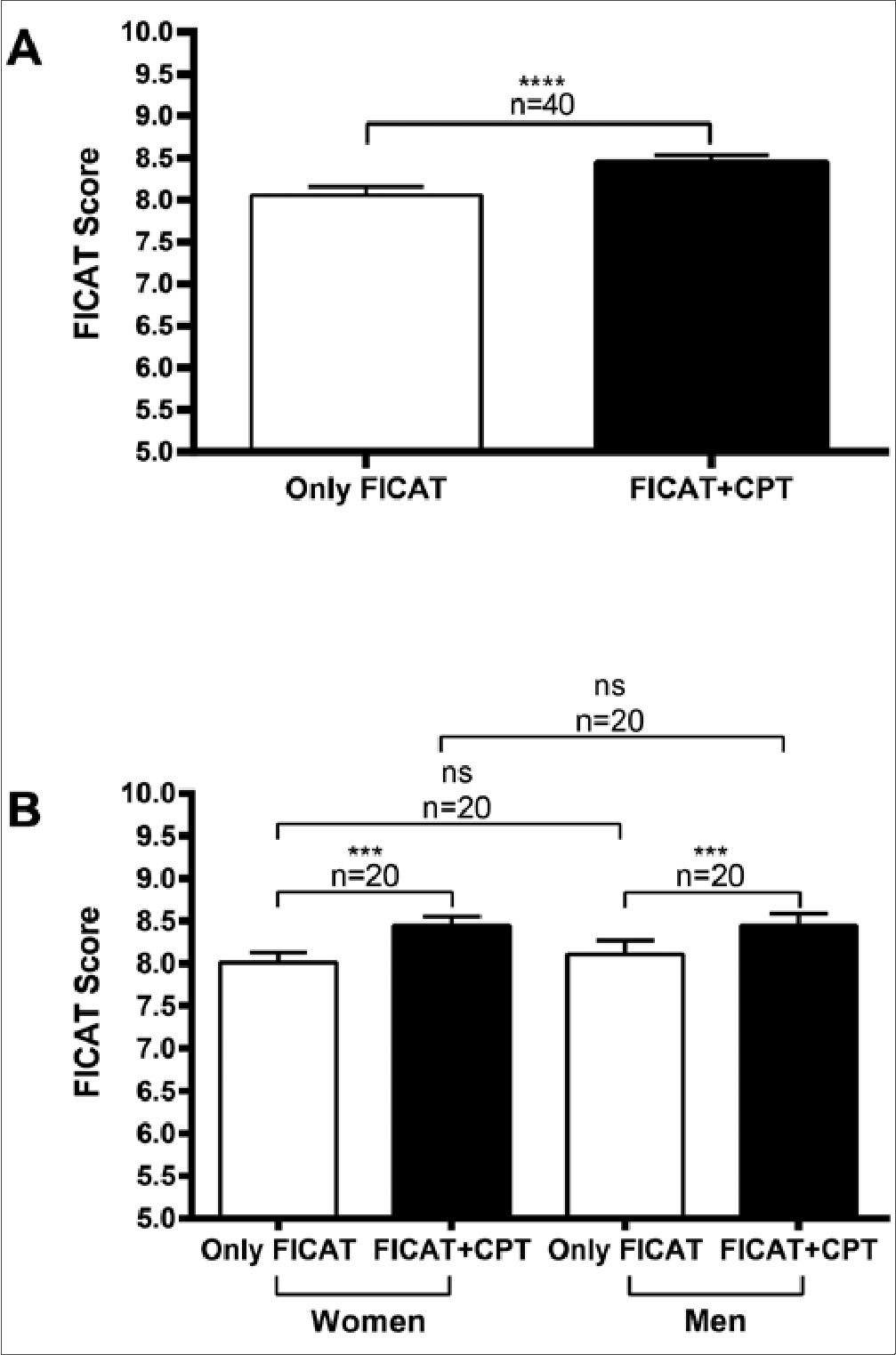
Figure 1. Effect of acute cold noxious stimulation on cognitive performance. The Flanker Inhibitory Control and Attention Test (FICAT) of the NIH Toolbox was applied alone, without any additional stimulation, or during the simultaneous application of the Cold Pressor Test (CPT). A. Score derived from the entire sample during pain-free (Only FICAT) or painful (FICAT+CPT) circumstances; B. Sex-based results. Columns represent the mean±standard deviation (***: p < 0.001; ****: p < 0.0001; ns: non-significant). The cognitive test evaluates the participant’s ability to deal with abundant environmental stimulation.
-
Results
-
Cognitive performance
As shown in Figure 1A, when participants (n = 40) were presented with FICAT and CPT simultaneously, their overall performance on the cognitive test increased significantly (p < 0.0001; 95% CI 0.1700 to 0.7100) compared to the control (pain-free) scenario (8.06 ± 0.09, Only FICAT vs. 8.45 ± 0.08, FICAT+CPT). A positive correlation (r=0.7936) was also obtained between cognitive performance and acute pain. These results indicate that despite the possible interference caused by the noxious situation, participants scored better on the cognitive test when simultaneously exposed to the experimentally induced acute pain condition. When analyzing performance about sex influences (Figure 1B), men and women showed a similar increase (p < 0.001; 95% CI for women: 0.1400 to 0.8400; for men: 0.2000 to 0.8100) in their cognitive performance when exposed to the concurrent FICAT and CPT (Women: 8.01 ± 0.12 vs. 8.45 ± 0.10, n = 20; Men: 8.11 ± 0.16 vs. 8.45 ± 0.14, n = 20; Only FICAT vs. FICAT+CPT, for both groups).
The FICAT score considers computed accuracy and reaction time. Given that all subjects obtained a perfect score (five points) under both experimental conditions (only FICAT, and FICAT+CPT), this factor did not influence the overall performance variations observed here. Therefore, we analyzed the influence of attention and inhibitory control on the reactiontime component. As shown in Figure 2A, all participants (n = 40) showed a significant decrease (p < 0.0001; 95% CI -0.1140 to -0.07710) in reaction time when simultaneously exposed to the cognitive test and the noxious stimulation, compared to scores in the pain-free scenario (0.81 ± 0.01 sec, Only FICAT vs. 0.71 ± 0.01 sec, FICAT+CPT). These results indicate that the participants responded faster despite the possible challenge to their attention and inhibitory control caused by acute noxious stimulation. Their performance on the cognitive test is also depicted in Figure 2B based on the type of stimulus (congruent vs. incongruent), considering incongruent stimuli as the most significant cognitive challenge. When subjects (n = 40) performed the test in the pain-free scenario (Only FICAT), they responded significantly slower (p < 0.001; 95% CI: 0.02064 to 0.07699) for incongruent stimuli (0.84 ± 0.01 sec, incongruent stimuli vs. 0.78 ± 0.01 sec, congruent stimuli). However, when they were simultaneously exposed to the cognitive test and acute pain stimulation (FICAT+CPT), their responses to both types of stimuli were significantly faster compared to the pain-free scenario (0.73 ± 0.01 sec, incongruent stimuli vs. 0.71 ± 0.01 sec, congruent stimuli; p < 0.0001; 95% CI congruent stimuli Only FICAT vs. congruent stimuli FICAT+CPT: -0.1099 to-0.06159; incongruent stimuli Only FICAT vs. incongruent stimuli FICAT+CPT: -0.1398 to -0.08192) but not significantly different from each other, suggesting that cold noxious stimulation appears to balance the cognitive load of the test.
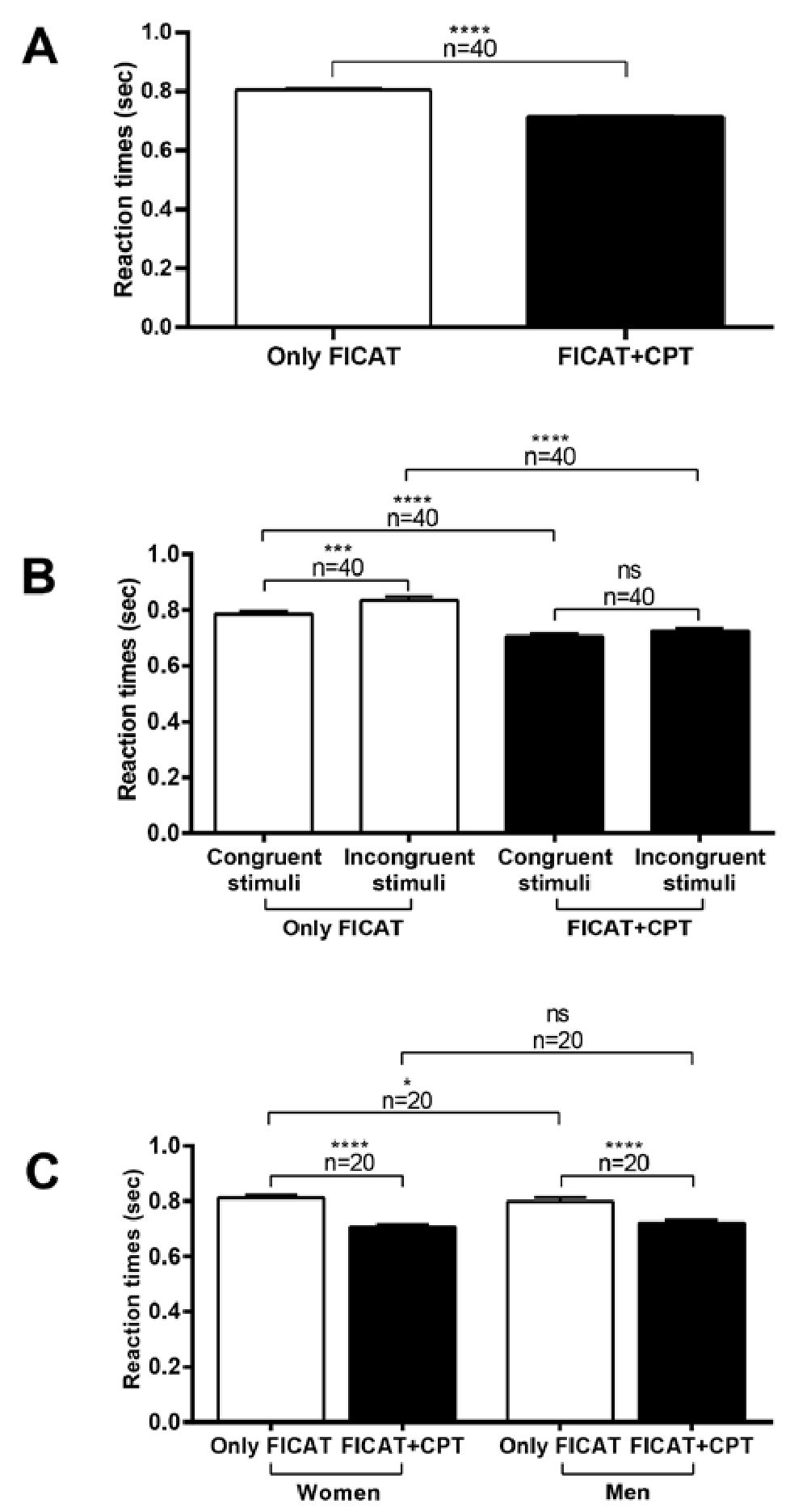
Figure 2. Cognitive modulation of pain promotes faster reaction times. Values (sec) were obtained when participants were exposed to either the cognitive test (Only FICAT) or the combined paradigm (cognitive test + acute cold, painful stimulation; FICAT+CPT). A. Variations in reaction time of the whole sample; B. Variations in reaction time variations concerning the type of stimulus (congruent vs. incongruent); C. Sexbased reaction time variations. Columns represent the mean±standard deviation (*: p < 0.05; ***: p < 0.001; ****: p < 0.0001; ns: nonsignificant; FICAT: Flanker Inhibitory Control and Attention Test; CPT: Cold Pressor Test).

Figure 3. A cognitive task can modulate tolerance to painful cold stimulation. Participants were exposed to the noxious stimulus (Only CPT) or the combined paradigm (acute painful stimulation + cognitive test; FICAT+CPT). A. Tolerance variations of the entire sample; B. Sex-related variations in tolerance to cold pain. Columns represent the mean±standard deviation (**: p < 0.01; ****: p < 0.0001; ns: nonsignificant; FICAT: Flanker Inhibitory Control and Attention Test; CPT: Cold Pressor Test).
The results shown in Figure 2C also reflect that the simultaneous application of FICAT+CPT significantly improves the cognitive performance of the subjects compared to the pain-free scenario (p < 0.0001; 95% CI for women: -0.1280 to -0.07907; for men: -0.1123 to -0.05698) (Women: 0.81 ± 0.01 sec vs. 0.71 ± 0.01 sec, n = 20; Men: 0.80 ± 0.01 sec vs. 0.72 ± 0.01 sec, n = 20; Only FICAT vs. FICAT+CPT, for both groups). When comparing the sexes, men responded slightly faster than women only in the absence of noxious stimulation (p < 0.05; Only FICAT; 95% CI: -0.1280 to -0.07907).
-
Pain tolerance
According to Figure 3A, participants significantly increased (p < 0.0001; 95%: 0.00013 to 32.00) their average pain tolerance when simultaneously exposed to cognitive challenge and CPT (49.15 ± 4.50 sec, Only CPT vs. 66.18 ± 3.96 sec, FICAT+CPT). It should be noted that sex variations in tolerance to painful stimulation were observed, at least initially (Figure 3B). Men (n = 20) had longer exposure times (p < 0.01; 95%
CI: 9.000 to 51.00) than women (n = 20) when exclusively subjected to the CPT (36.20 ± 5.63 sec, Women vs. 62.10 ± 5.82 sec, Men). On the contrary, when women were exposed simultaneously to the cognitive challenge and the noxious stimulation, there was a very significant increase (p<0.0001; 95% CI: 0.00012 to 36.00) in their tolerance to the noxious stimulus (36.20 ± 5.63 sec, Only CPT vs. 58.95 ± 5.41 sec, FICAT+CPT), as compared to the tolerance shown by men (62.10 ± 5.82 sec, Only CPT vs. 73.40 ± 5.43 sec, FICAT+CPT; p < 0.01; 95% CI: 0.00010 to 32.00).
-
Pain intensity
Changes in the perception of pain intensity under the different experimental conditions are shown in Figure 4A. As expected, during the baseline period (no stimulation at all) and the performance of the cognitive test (Only FICAT), participants (n = 40) did not report changes in pain intensity. However, a significant (p < 0.0001; 95% CI: 4.600 to 6.200) change in pain score was observed under acute cold pain (Baseline vs. Only CPT). Interestingly, the intensity of pain was significantly higher (p < 0.001; 95% CI: 0.2000 to 1.900) when noxious stimulation was applied simultaneously with the cognitive test (5.50 ± 0.28, Only CPT vs. 6.51 ± 0.30, FICAT+CPT).
As shown in Figure 4B (n = 40), the increases in pain intensity during the combined condition were accompanied by a significant increase (p < 0.0001; 95% CI: 0.0001 to 32.00) of tolerance to noxious stimulation (49.15 ± 4.50 sec, Time CPT vs. 66.18 ± 3.96 sec, Time FICAT+CPT), suggesting that the cognitive task could have generated a distracting effect on pain perception. Lastly, as indicated in Figure 4C, participants of both sexes reported higher pain intensity scores during exposure to the combined condition (Women: 5.58±0.41 vs. 7.04 ± 0.34, n = 20; Men: 5.41 ± 0.39 sec vs. 5.98 ± 0.47 sec, n = 20; Only CPT vs. FICAT+CPT, for both groups). However, this trend was statistically significant (p<0.01; 95% CI: 0.3000 to 2.550) exclusively for women.
-
Discussion
Pain should be considered a complex experience shaped by psychological factors[2],[6] and not merely a reflection of nociceptive input. Despite numerous studies on attention and pain, there is yet to be a consensus on the characteristics that determine how much pain interferes with task performance and vice versa[18].
Our results indicate improvements in cognitive performance, particularly in attention and inhibitory control, and increased tolerance to pain when cold noxious stimulation was applied during the administration of a cognitive test. These results provide additional evidence of a deviation of attentional focus, initially directed to pain, towards a circumstance that requires a certain level of cognitive effort. This active approach of distraction[8] can be interpreted as a cognitive coping strategy that includes a more complex interaction with a distractor (in our case, the FI- CAT) and the subject’s active role during a painful situation. This performance improvement was also reflected in the decreased reaction times to congruent and incongruent stimuli despite the possible distraction caused by the acute pain condition.
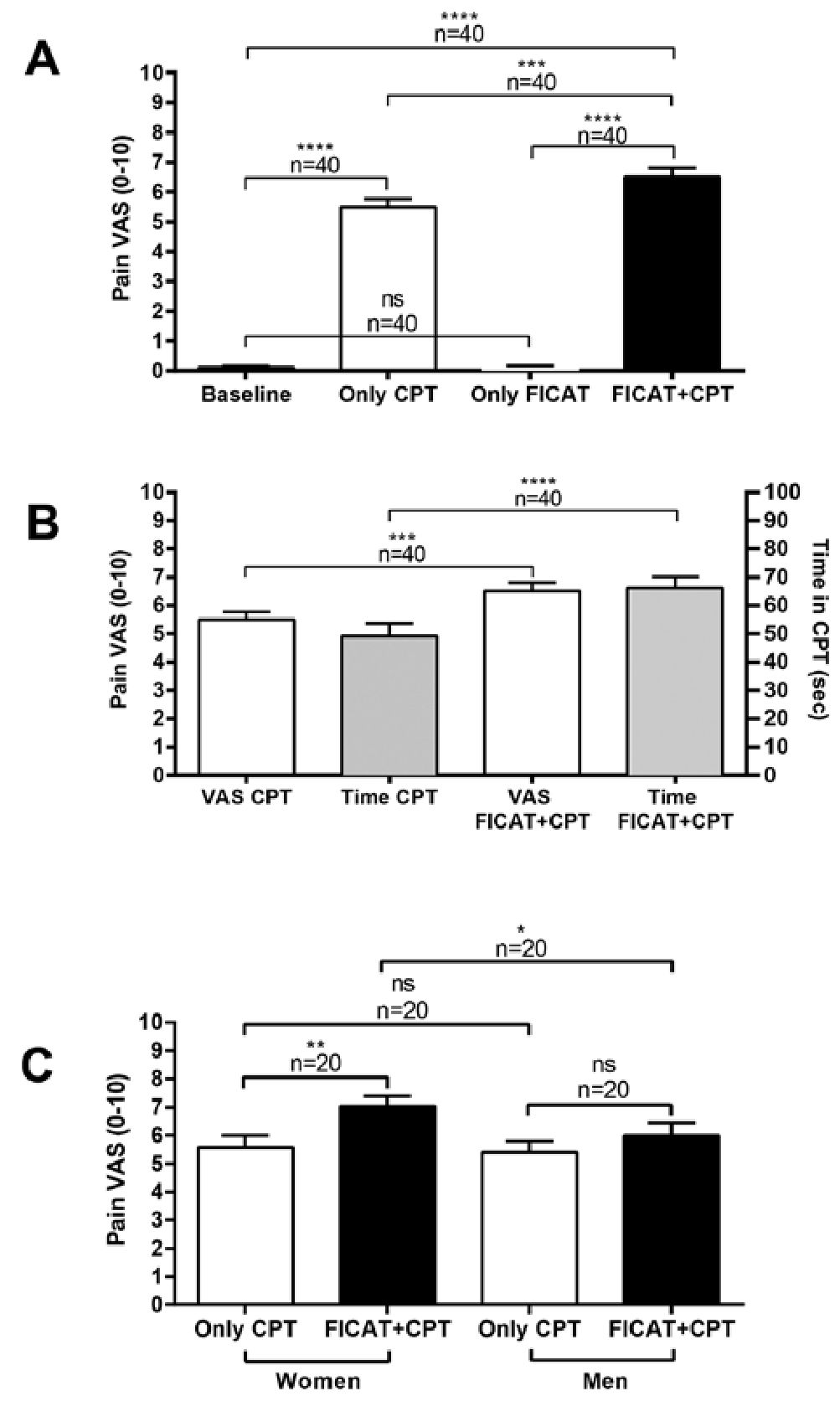
Figure 4. Cognitive modulation preferentially modulates pain tolerance rather than pain perception. A. Changes in the perception of cold pain under different experimental conditions; B. Comparison of intensity and tolerance to cold painful stimulation in different scenarios. C. Sex- related variations in the intensity of cold pain. Columns represent the mean±standard deviation (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001; ns: non-significant; VAS: Visual Analogue Scale; FI- CAT: Flanker Inhibitory Control and Attention Test; CPT: Cold Pressor Test).
A significant correlation was reported between efficient cognitive inhibitory control and faster response times while performing a cognitive test[15], indicating better executive functioning abilities. Our results showed a significantly improved tolerance to acute pain when faced with a cognitive challenge, particularly in women, that may have been influenced by attention and inhibitory control.
Longer exposure time to CPT, reduced pain intensity and unpleasantness ratings have been associated with better outcomes on the Stroop test[19], which also evaluates inhibitory control. Furthermore, neuroimaging assessments have shown common neural pathways for attention and endogenous pain inhibitory mechanisms that can reciprocally modulate each other[20]-[22]. Areas such as the prefrontal cortex (specifically the dorsolateral and medial regions), anterior cingulate cortex, and periaqueductal gray matter have been identified in the cognitive modulation of pain[3],[20],[23]-[25]. Previous studies have indicated that projections originating from these brain regions converge and add to the descending endogenous modulatory system, which regulates the ascending traffic of nociceptive information to generate analgesia[20]. Thus, alterations in the anatomical integrity and functioning of the brain regions involved in pain control and cognitive functioning could explain why long-term chronic pain patients develop cognitive deficits and how a cognitive task may modulate the endogenous capacity to control and tolerate pain situations.
While the average pain tolerance increased during the simultaneous performance of the CPT and the cognitive test, most participants in this condition also reported higher levels of pain intensity, especially in women. Previous evidence on cognition and pain indicates that cognitive challenges are required to alter the perception of intensity of pain[1],[26]. Two research groups[18],[27] used experimental designs with different degrees of cognitive test complexity during CPT applications. Study participants reported lower pain intensity scores only when exposed to tasks that required a high level of cognitive performance. Furthermore, it has been proposed that to change pain perception, the cognitive challenge needs to be difficult, as well as the level of pain[18]. In this study, the CPT was sufficiently intense according to our VAS scores, but the cognitive load was potentially not severe enough to divert attention away from pain perception. However, as previously suggested[6],[7], it is unlikely that pain attention can be fully inhibited due to the uncomfortable experience of noxious stimulation.
On the other hand, an increase in CPT exposure time is expected to be accompanied by a certain degree of skin hypersensitivity, at least temporarily. Skin hypersensitivity after cold is a well-documented phenomenon[28] and might represent a trigger factor for pain induced, even when stimulation is absent.
The severity of pain from immersion in cold water has been inversely related to temperature, but is directly related to the duration of immersion time[29]. As soon as the hand is immersed, there is an initial sensation of cold followed by an aching or crushing pain that rapidly increases in intensity, reaching a maximum at 60-90 seconds. This increase is followed by adaptation when the pain subsides four to five minutes later and the pain is no longer perceived[28],[29]. Due to the application of CPT, the absence of the expected inhibition, usually exerted by non-nociceptive sensory neurons on nociceptive pathways, may cause peripheral sensitization and, consequently, central sensitization at the spinal cord level[28].
Another possible explanation for the increase in pain perception might be related to the time pain perception was requested[30]. The VAS score was required immediately after the cognitive challenge was terminated rather than periodically during its application to avoid interference with the cognitive test. At that moment, the executive challenge was no longer acting, which (during the combined paradigm) could result in a “redirection” of attention to pain, affecting overall perception of pain intensity, as previously suggested[23].
-
Conclusion
Our results indicate that it is possible to actively increase tolerance to acute cold pain if attention is directed toward a cognitive stimulus. Exposure to a painful stimulus (cold temperature) should not imply “a priori” a disruption in the level of attention and the ability to exert inhibitory control. In addition, executive capacity can be improved in acutely painful conditions. Our findings also suggest that a cognitive task could increase pain tolerance, acting as a complementary strategy to traditional analgesic therapy, for example, serving as an alternative for patients with allergic, intolerant, or refractory afflictions. However, more research is needed to determine the optimal level of cognitive load necessary to promote a reduction in pain intensity.
These results also led us to consider whether a patient’s pain severity may be underestimated when attention is sufficiently distracted by cognitively demanding activities or even during the anamnesis process, evoking a transitory nociceptive-masking effect.
Acknowledgments: We want to thank Empresas Polar, Caracas, Venezuela, for the generous donation of four 6th-gener- ation iPads, and Yarfraz Nazuddeen for his technical support.
Ethics:
The study has not been sent to another national or international scientific journal.
The authors declare that they have obtained the approval of the appropriate institutional review board and have followed the ethical principles outlined by the World Medical Association and the Declaration of Helsinki for human experimental investigations. Furthermore, informed consent was obtained from all participants before participation, and their identities were protected throughout. This work was partly supported by the UNIMET Research and Development Directorate, which is attached to the Deanship of Research and Development.
Conflicts:
The authors declare that they have no conflict of interest and transfer the intellectual property rights of the article to the Chilean Journal of Anesthesiology.
-
References
1. Moore DJ, Eccleston C, Keogh E. Cognitive load selectively influences the interruptive effect of pain on attention. Pain. 2017 Oct;158(10):2035-41. https://doi.org/10.1097/j.pain.0000000000001011.
2. Moriarty O, Finn DP. Cognition and pain. Curr Opin Support Palliat Care. 2014 Jun;8(2):130–6. https://doi.org/10.1097/SPC.0000000000000054 PMID:24722475
3. Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008 Aug;12(8):306–13. https://doi.org/10.1016/j.tics.2008.05.005 PMID:18606561
4. Bingel U, Tracey I, Wiech K. Neuroimaging as a tool to investigate how cognitive factors influence analgesic drug outcomes. Neurosci Lett. 2012 Jun;520(2):149-55. . neulet.2012.04.043 PMID:22561551 https://doi.org/10.1016/j.neulet.2012.04.043.
5. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013 Jul;14(7):502–11. https://doi.org/10.1038/nrn3516 PMID:23719569
6. Eccleston C. Chronic pain and distraction: an experimental investigation into the role of sustained and shifting attention in the processing of chronic persistent pain. Behav Res Ther. 1995 May;33(4):391–405. https://doi.org/10.1016/0005-7967(94)00057-Q PMID:7538753
7. Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999 May;125(3):356–66. https://doi.org/10.1037/0033-2909.125.3.356 PMID:10349356
8. Fernández E. A classification system of cognitive coping strategies for pain. Pain. 1986 Aug;26(2):141–51. https://doi.org/10.1016/0304-3959(86)90070-9 PMID:3531980
9. Terrighena EL, Shao R, Lee TM. Impact of concurrent cognitive processing on cold pain perception: implications for pain management and its neurobiological basis. Appl Neuropsychol Adult. 2017;24(1):81–91. https://doi.org/10.1080/23279095.2015.1100618 PMID:27078504
10. Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013 Mar;80(11 Suppl 3):S2–6. https://doi.org/10.1212/WNL.0b013e3182872e5f PMID:23479538
11. Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Slotkin J, et al. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc. 2014 Jul;20(6):567–78. https://doi.org/10.1017/S1355617714000320 PMID:24959840
12. National Institutes of Health and Northwestern University. NIH Toolbox Flanker Inhibitory Control and Attention Test Technical Manual. 2012. Available: https://www.healthmeasures.net/im-ages/nihtoolbox/Technical_Manuals/Cognition/Toolbox_Flanker_Inhibitory_Control_and_Attention_Test_Technical_Manual.pdf. (Accessed 3 January 2024).
13. Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013 Mar;80(11 Suppl 3):S54–64. https://doi.org/10.1212/WNL.0b013e3182872ded PMID:23479546
14. Mitchell LA, MacDonald RA, Brodie EE. Temperature and the cold pressor test. J Pain. 2004 May;5(4):233–7. https://doi.org/10.1016/j.jpain.2004.03.004 PMID:15162346
15. Kerlinger FN, Lee HB. Foundations of Behavioral Research. 4th ed. Fort Worth, TX: Harcourt College Publishers, 2000 p.
16. The World Medical Association (WMA). Declaration of Helsinki. 2014. Available: https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/. (Accessed 15 December 2023).
17. International Association for the Study of Pain (IASP). Ethical Guidelines for Pain Research in Humans. n.d. Available: https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1213. (Accessed 15 December 2023).
18. Veldhuijzen DS, Kenemans JL, de Bruin CM, Olivier B, Volkerts ER. Pain and attention: attentional disruption or distraction? J Pain. 2006 Jan;7(1):11-20. https://doi.org/10.1016/j.jpain.2005.06.003
19. Oosterman JM, Dijkerman HC, Kessels RP, Scherder EJ. A unique association between cognitive inhibition and pain sensitivity in healthy participants. Eur J Pain. 2010 Nov;14(10):1046–50. https://doi.org/10.1016/j.ejpain.2010.04.004 PMID:20493746
20. Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002 Feb;125(Pt 2):310–9. https://doi.org/10.1093/brain/awf022 PMID:11844731
21. Dreher JC, Berman KF. Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci USA. 2002 Oct;99(22):14595–600. https://doi.org/10.1073/pnas.222193299 PMID:12391312
22. Legrain V, Bruyer R, Guérit JM, Plaghki L. Involuntary orientation of attention to unattended deviant nociceptive stimuli is modulated by concomitant visual task difficulty. Evidence from laser evoked potentials. Clin Neurophysiol. 2005 Sep;116(9):2165–74. https://doi.org/10.1016/j.clinph.2005.05.019 PMID:16055373
23. Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA. 2013 Nov;110(46):18692–7. https://doi.org/10.1073/pnas.1312902110 PMID:24167282
24. Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002 Apr;22(7):2748–52. https://doi.org/10.1523/JNEUROSCI.22-07-02748.2002 PMID:11923440
25. Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain. 2004 Jun;109(3):399–408. https://doi.org/10.1016/j.pain.2004.02.033 PMID:15157701
26. Moore DJ, Keogh E, Eccleston C. The interruptive effect of pain on attention. Q J Exp Psychol (Hove). 2012;65(3):565–86. https://doi.org/10.1080/17470218.2011.626865 PMID:22136653
27. Torta DM, De Laurentis M, Eichin KN, von Leupoldt A, van den Broeke EN, Vlaeyen JW. A highly cognitive demanding working memory task may prevent the development of nociceptive hypersensitivity. Pain. 2020 Jul;161(7):1459–69. https://doi.org/10.1097/j.pain.0000000000001841 PMID:32102023
28. Foulkes T, Wood JN. Mechanisms of cold pain. Channels (Austin). 2007;1(3):154–60. https://doi.org/10.4161/chan.4692 PMID:18690033
29. Kreh A, Anton F, Gilly H, Handwerker HO. Vascular reactions correlated with pain due to cold. Exp Neurol. 1984 Sep;85(3):533–46. https://doi.org/10.1016/0014-4886(84)90029-3 PMID:6468576
30. Hirsch MS, Liebert RM. The physical and psychological experience of pain: the effects of labeling and cold pressor temperature on three pain measures in college women. Pain. 1998 Jul;77(1):41–8. https://doi.org/10.1016/S0304-3959(98)00080-3 PMID:9755017

 ORCID
ORCID