Aurio Fajardo-Campoverdi MD. MSc. MEC. PhD.(c).1, Fernando Paziencia2, Carmen Chica-Meza Ft. Mg.3, Yolanda López-Fernández MD. PhD.4, Miguel Ibarra-Estrada MD.5, Angelo Roncalli-Rocha Pt. MSc.6, Enrique Monares-Zepeda MD.7, Alejandro González-Castro MD, PhD.8, Alberto Medina MD, PhD. PICU.9, Vicent Modesto i Alapont MD, PhD. PICU.10, Adlay Martínez-Ramos MD.11, from the International Group of Mechanical Ventilation (WeVent).
Recibido: 08-04-2024
Aceptado: 09-06-2024
©2024 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 53 Núm. 5 pp. 509-519|https://doi.org/10.25237/revchilanestv53n5-11
PDF|ePub|RIS
Diagnóstico y soporte ventilatorio del SDRA en adultos: una encuesta internacional
Abstract
Background: The current definition of acute respiratory distress syndrome (ARDS) is undergoing multiple revisions and is the subject to international debate about its validity and applicability. Methods: Analysis of the international survey addressed to all health personnel in charge of critical patients. This survey consists of twenty-two questions, in three languages . The aim was quantifying the perspectives related to the definition, diagnosis and ventilatory support of ARDS. Results: A total of 346 surveys answered worldwide from 34 countries were analyzed. The country that contributed most was Colombia (24.9%). The lowest acceptable PaO2 for ARDS was between 86 to 90 mmHg (52.6%; 95% CI: 163.7 to 200.3). For 67.3% (95% CI: 215.8 to 250.2) the initial ventilatory mode for patients with moderate to severe ARDS is volume-controlled ventilation. 39% (95% CI: 117.1 to 152.9) responded that they use the FiO2/PEEP table in ARDS for positive end-expiratory pressure (PEEP) titration. 73.1% (95% CI: 236.8 to 269.2) believe that it is necessary to incorporate the SpO2/FiO2 parameter to define ARDS. While 59.3% (95% CI: 186.9 to 223) did not use Mechanical Power (MP) as a safety parameter for mechanical ventilator setting. Finally, 32.4% (95% CI 52.8 to 79.2) of respondents believe that a safe level of MP to minimize ergotrauma is up to 15 J/min. Conclusions: The intervention guidelines and practice patterns reported by respondents, associated with diagnosis and ventilatory strategies in ARDS patients, differ widely among different critical care health professionals, even within professionals of the same group.
Resumen
Introducción: La definición actual del Síndrome de Distrés Respiratorio Agudo (SDRA) está siendo sometida a múltiples revisiones y es objeto de debate internacional respecto de su validez y aplicabilidad. Métodos: Análisis de una encuesta internacional dirigida a todo el personal sanitario a cargo de pacientes críticos. Esta encuesta consta de 22 preguntas, en 3 idiomas. El objetivo principal fue cuantificar las distintas perspectivas relacionadas con la definición, el diagnóstico y el soporte ventilatorio en el SDRA. Resultados: Se analizaron 346 encuestas contestadas procedentes de 34 países a nivel mundial. El país que más contribuyó fue Colombia (24,9%). La PaO2 más baja aceptable para el SDRA se situó entre 86 y 90 mmHg (52,6%; IC 95%: 163,7 a 200,3). Para el 67,3% (IC 95%: 215,8 a 250,2) el modo ventilatorio inicial, para pacientes con SDRA moderado a severo, es la ventilación controlada por volumen. El 39% (IC del 95%: 117,1 a 152,9) respondió que utiliza la tabla FiO2/PEEP para la titulación de la presión positiva al final de la espiración (PEEP). El 73,1% (IC 95%: 236,8 a 269,2) cree que es necesario incorporar el parámetro SpO2/FiO2 en la definición del SDRA. El 59,3% (IC 95%: 186,9 a 223) no utiliza la Potencia Mecánica (PM) como parámetro de seguridad para el ajuste del ventilador mecánico durante la asistencia ventilatoria del SDRA. Por último, el 32,4% (IC 95%: 52,8 a 79,2) de los encuestados cree que un nivel seguro de PM para minimizar el ergotrauma es de hasta 15 J/min. Conclusiones: Las pautas de intervención y los patrones de práctica comunicados por los encuestados, asociados al diagnóstico y a las estrategias ventilatorias en pacientes con SDRA, difieren ampliamente entre los distintos profesionales sanitarios de cuidados críticos, incluso dentro de profesionales de un mismo grupo.
Key messages
| 1. The current definition of ARDS has multiple interpretations over time; however, even despite international expert consensus, the perception of healthcare personnel in charge of critically ill patients is very heterogeneous. |
| 2. The guidelines for ventilatory management in patients with ARDS appear to be a rather homogeneous consolidation, although there is variability among different professionals in the ICU. |
-
Introduction
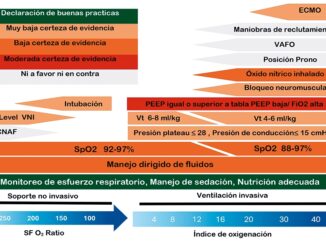
The first approach to the definition of acute respiratory distress syndrome (ARDS) was made in the 1960’s by Dr. David Ashbaugh et al[1]. In this definition, this syndrome was characterized through ventilatory variables such as respiratory system distensibility (Crs), partial pressure of carbon dioxide in arterial blood (PaCO2) and partial pressure of oxygen in arterial blood (PaO2) levels, respiratory rate (RR) and the PiO2-PaO2 gradient.
In the late 80’s, Murray et al.[2], maintained the variable of distensibility within the previously proposed definition, correctly contributing the acute appearance of new interstitial infiltrates or pulmonary consolidations in the chest X-ray. He also classified severity according to the levels of positive end-expiratory pressure (PEEP) required and for the first time the PaO2/FiO2 (P/F) ratio appeared as a variable for classifying the severity of hypoxemia. It was not until the 90’s, with the creation of the American-European Consensus[3], when pulmonary artery wedge pressure measurement was included in the definition of acute lung injury (ALI) (< 300 mmHg + PEEP) differentiating it from ARDS through P/F ratio values (< 200 mmHg + PEEP). The Berlin Consensus[4]of 2012 remained in force for many years. It tried to be more specific in the definition:
1. Considering acuity as clinical onset or worsening occurring in the last 7 days.
2. The importance of use of chest X-ray to evaluate opacities
not caused by pulmonary edema of cardiogenic origin.
3. Finally, the most significant contribution was the classification of severity by maintaining the P/F ratio:
Mild < 300 to 200 mmHg + PEEP or CPAP ≥ 5 cmH2O
Moderate ≤ 200 to 100 mmHg + PEEP or CPAP ≥ 5 cmH2O)
Severe ≤ 100 mmHg + PEEP or CPAP ≥ 5 cmH2O
Currently, several revisions and updates have been postulated to redefine this syndrome, being that these proposals are based on expert opinions[5],[6]. In this context, it seems reasonable to consider the need to be able to objectify and quantify the perspectives and judgments of professionals and experts. The present investigation is based on the relevance of the survey previously conducted by Dr. Carmichael et al[6]in 1996. The survey conducted by Dr. Carmichael made it possible to objectively and systematically quantify the perceptions of health professionals, thus providing a more concrete and comprehensive view of the various existing perspectives. The main objective was to explore and measure the opinions of experts in critical medicine and critical care regarding ARDS and associated ventilatory support. The quantitative approach used in the baseline survey allowed the collection of robust data, which allowed inferences to be generated through risk analysis. Secondary objectives were to establish a comparative relationship between the baseline survey and the present knowledge from health professionals involved in intensive care units (ICU).
-
Materials and Methods
We conducted a cross-sectional observational analytical study, through the design and execution of an international survey in three languages (Spanish, English and Portuguese) aimed at all healthcare personnel in charge of critical patients. This survey consisted of twenty-two questions with the aim of quantifying the different opinions related to the definition, diagnosis and support of patients with ARDS.
The current Berlin definition of ARDS was used, as well as the severity classification of ARDS. The survey design was conceived as a pilot, which was conducted among 13 critical care and critical care experts from different disciplines and countries. Once this pilot was completed, revisions and corrections were made, estimating a maximum time of 15 minutes for completion. The types of questions included were multiple choice, single response and open-ended questions to collect categorical information on mechanical ventilation and ARDS.
Inclusion criteria (potential respondents) were considered to be all health professionals in charge of critical patients in an ICU, regardless of their specialty (or even in the absence of specialty) and the type of critical unit they were in charge of.
After that, we proceeded to carry out the dissemination. This survey was conducted in electronic format using Google Forms and was promoted and disseminated through horizontal, vertical and mixed social networks, via e-mail and websites and/or applications in a random manner. Structural development and data collection took place between March 7 and April 21, 2023 and it was anonymous and voluntary. The participants had to give their informed consent. This was an express consent, based on a positive action on the part of the respondents who participated in this study, in which they accepted the conditions of the study, as well as the privacy policy, and was assumed by answering the questions and sending the electronic document to the online database. This study received the approval of the Ethics Committee of the ESC, which met on January 17, 2023, by means of ACTA #9, resolution 8430 of 1993.
-
Professionals
To broaden the professional horizon to a multidisciplinary level, the following categories were considered: ICU medical specialist staff (IMS), ICU medical non-specialist staff (IMN-S) which includes physicians with other specialties, kinesiologist or physiotherapist (Kine/Pt) (for this study, they were defined as equivalent since in both cases they are health professionals with at least 5 years of university studies), respiratory therapist (Rt) (defined as graduates in respiratory care, but which do not correspond to university studies) and the category “other” (general practitioners, inhalation therapists, nurses).
-
Statistical analysis
An initial descriptive analysis was made of the variables considering demographic, whose values are expressed as proportions and their respective confidence intervals (95%).
The comparison between two polytomous variables was performed using the Chi-square test, and with those variables that presented significant association, multinomial regressions were performed to calculate margins and subsequently plot the associated risk.
For the categorical response variables, multiple binomial regressions were used, and the profession variable was used as the independent variable (dummy), expressing the results as relative risk (RR) with their respective confidence intervals. Similarly, with those variables with significant association, multinomial regressions were performed to calculate margins and subsequently plot the associated risk. For continuous variables, multiple simple linear regressions were used to calculate the mean difference between the significant variables for linear post-estimation. Finally, the margins were calculated and the associated risk was plotted. STATA v.18 (StatsCorp, College Station, TX, USA) was used for data analysis and the significance level was 0.05 (two-tailed test).
-
Results
-
Demographics
A total of 346 responses were collected internationally from 34 countries. The country with the highest response rate was Colombia (24.9%), followed by Mexico (17.1%), Brazil (11.9%) and Chile (10.1%). After categorization by continent, 214 (61.9%; 95% CI: 196.2 to 231.8) surveys corresponded to South America, and Africa contributed only 1 (0.3%; 95% CI: -0.9 to 2.9). The professionals who answered the most surveys were Kine/Pt with 35.3% (95% CI: 104.5 to 139.5), of which 56.5% (119 to 122.9) belong to South America, followed by IMS with 31.5% (95% CI: 91.9 to 126) of which 53.7% (95% CI: 33.8 to 54.2) from Central America. The difference between both polytomous variables (professionals and continent) was statistically significant (p < 0.01).
Regarding the years of experience of the professionals, the most prevalent category corresponds to < 5 years with 32.1% (95% CI: 93.9 to 128.1) and of which 41.4% (95% CI: 35.4 to 56.6) corresponds to Kine/Pt. The lowest prevalence category was between 10 to 15 years of experience with 18.2% (95% CI: 48.9 to 77.1) of which 34.9% (95% CI: 13.7 to 30.3) corresponded to IMS [Figure 1A]. The difference between years of experience regarding the different health care professionals was statistically significant (p < 0.05). The 53.8% (167.7 to 204.3) of professionals who responded to the survey reported employment in public hospitals and 79.2% (95% CI: 259.1 to 288.9) corresponded to critical care units classified as general ICU. The other demographic variables are specified in Table 1 and 1A.
-
ARDS
32.1% (95% CI: 93.9 to 128.1) of health care professionals believe that three criteria are needed to define ARDS, of this percentage, 37.8% (95% CI: 31.8 to 52.2) are IMS and Kine/ Pt. Of the respondents, 11.6% (95% CI: 28.3 to 51.7) believed that only one criterion was needed to define ARDS, and of this percentage, 32.5% (95% CI: 6.3 to 19.7) corresponded to IMS and 30% (95% CI: 5.5 to 18.5) to Kine/Pt. The difference between criteria for defining ARDS regarding the different health care professionals was not statistically significant (p = 0.09) (Table 1A).
For the question: What is the lowest acceptable PaO2 in your ICU for ARDS? 52.6% (95% CI: 163.7 to 200.3) answered between 86 to 90 mmHg, of which 36.3% (95% CI: 53.2 to 78.8) corresponded to IMS, 34.1% (95% CI: 49.3 to 74.6) to Kine/Pt, 18.9% (95% CI: 23.6 to 44.4) to Rt, 9.3% (95% CI: 9.2 to 24.8) to others and 1.7% (95% CI: -0.4 to 6.4) to IMN-S. Likewise, 1.2% (95% CI: 0.1 to 7.9) of the respondents believed that the minimum PaO2 should be < 75 mmHg, while 0.3% (95% CI: -0.9 to 2.9) believed it should be > 96 mmHg. The difference between these polytomous variables was statistically significant (p < 0.05) and the analysis of probability of choice of different PaO2 levels according to type of health care professionals is shown in Figure 2A. The rest of the analysis for this question is in Table 2A.
Regarding the initial ventilatory mode for patients with moderate to severe ARDS, 67.3% (95% CI: 215.8 to 250.2) preferred volume-controlled(VC) ventilation, of which 45.5% (95% CI: 90.9 to 121) corresponded to Kine/Pt and 30.5% (95% CI: 57.1 to 84.9) corresponded to IMS [Figure 2A]. On the other hand, 17.3% (95% CI: 46.1 to 73.9) responded that they preferred pressure-controlled (PC) ventilation, of which 38.3% (95% CI: 15.4 to 30.6) corresponded to IMS and 28.3% (95% CI: 9.9 to 24) to Rt [Figure 2A]. The difference between the polytomous variables (the professions and ventilatory mode) was statistically significant (p < 0.01) (Table 2A) Figure 1 shows the probability of choice of these ventilatory modes, according to profession.
Regarding the choice of tidal volume (Vt), 56.1% (95% CI: 175.8 to 212.2) chose between 6 to 8 ml/kg ideal weight, of which, 31.9% (95% CI: 49.2 to 74.8) corresponded to Kine/
Pt, 31.4% (95% CI: 48.2 to 73.8) corresponded to IMS, 23.7% (95% CI: 34.3 to 57.7) to Rt, 11.9% (95% CI: 14.1 to 32) to others and only 1% (95% CI: -0.8 to 4.8) to IMN-S. The difference between Vt choice regarding the different health care professionals was not statistically significant (p = 0.14) (Table 2A).

Figure 1. Probability of choice of ventilatory mode according to the type of professional in charge.
For the question: Do you consider that airway pressure influences the choice of Vt? 84.9% (95% CI: 280.1 to 307.1) answered “Yes”, of which the highest proportion corresponded to Kine/Pt with 32.7% (RR 0.87; 95% CI: 0.75 to 1; p = 0.06). However, there was no statistically significant difference in relation to any of the professionals (Table 2).
Regarding the question: Do you intentionally allow CO2 retention in ARDS? 76.9% (95% CI: 250.6 to 281.4) answered “Yes”, where 35.3% (RR 1.97; 95% CI: 1.32 to 2.94); p < 0.01) corresponded to IMS and 38.4% (RR 1.91; 95% CI: 1.28 to 2.85); p < 0.01) corresponded to Kine/Pt. For the rest of the professionals the values were not statistically significant (Figure 2).
Regarding the question that addresses the issue of whether or not to use the FiO2/PEEP table in ARDS for PEEP titration, 39% (95% CI: 117.1 to 152.9) answered “Yes”, although only for the group of Kine/Pt was it statistically significant with
Table 1. Demographic variables (baseline)

Description of the demographic variables of the study. The data are expressed in number, their respective percentage and 95% confidence interval (CI).
IMS, ICU medical specialist staff; IMN-S, ICU medical non-specialist staff; Kine/Pt, kinesiologist and physiotherapist.
21.5% (RR 0.51; 95% CI: 0.31 to 0.83; p < 0.01). It should be clarified that 31.1% belonging to Rt was the group that showed the highest probability of risk for the use of this table, although it was not statistically significant (RR 1.16; 95% CI: 0.31 to 0.83; p = 0.48) (Figure 2).
For the question: do you use the driving pressure (DP) as a safety parameter for mechanical ventilator adjustment? 91% (95% CI: 304.5 to 325.5) answered “Yes”, and again the Kine/ Pt group with 37.5% (RR 1.29; 95% CI: 1.05 to 1.58; p < 0.05) was the only statistically significant variable (Figure 2).
In the context of the question related to the inclusion of the S/F parameter to define ARDS, 73.1% (95% CI: 236.8 to 269.2) agreed with doing so, however, it was not significant for the categorization according to the type of professional (Table 2).
Likewise, on the topic of whether or not to include high- flow nasal cannula (HFNC) in the definition of ARDS, 50.3% (95% CI: 155.7 to 192.3) were not in favor of doing so. Of these, 27.3% (RR 0.68; 95% CI: 0.47 to 1; p = 0.05) belonged to the professional group of Kine/Pt (Figure 2).
On the other hand, the survey results report that 59.3% (95% CI: 186.9 to 223) do not use Mechanical Power (MP) as a safety parameter for mechanical ventilator adjustment, and for this group the difference between the different health care professionals was not significant (Table 2).
In the question: What is the highest level of PEEP you have used? the results show that 43.3% (95% CI: 130.9 to 167.1) answered between 10 to 15 cmH2O, followed by 39.8% (95% CI: 119.1 to 154.9) between 16 to 20 cmH2O. IMS presented an average difference of 2.5 cmH2O of PEEP (95% CI: 0.81 to 4.18; p < 0.01) more than the group of other professionals, while Kine/Pt presented an average difference of 2.4 cmH2O of PEEP (95% CI: 0.69 to 4.01; p < 0.01) more than the group of other professionals, both differences being statistically significant (Figure 3). In turn, the difference between IMS and Kine/Pt was 0.13 cmH2O of PEEP (95% CI: – 0.96 to 1.22; p = 0.81). The other data associated with this question are detailed in Table 3.
Table 2. Determination of relative risk (RR) according to profession

Paw, airway pressure; VT, tidal volume; CO2, carbon dioxide; ARDS, acute respiratory distress syndrome; FiO2, fraction inspired oxygen; PEEP, positive end-expiratory pressure; DP, driving pressure; MV, mechanical ventilator; SpO2, peripheral oxygen saturation; HFNC, high-flow nasal cannula; MP, mechanical power.
IMS, ICU medical specialist staff; IMN-S, ICU medical non-specialist staff; Kine/Pt, kinesiologist and physiotherapist.
N, prevalence; RR, relative risk; CI, confidence interval.
The results presented are based on group 5 (other professionals) as the comparator in the analysis. Values in bold are statistically significant.
In the question: What PEEP level would you never exceed? 49.2% (95% CI: 135.6 to 170.4) answered between 16 to 20 cmH2O, followed by 23.8% (95% CI: 59.2 to 88.8) indicating that they would never exceed values between 10 to 15 cmH2O. The only professionals who presented a significant difference were the Kine/Pt with a difference in PEEP average of 3.2 cm- H2O (95% CI: 0.92 to 5.58; p < 0.01) with respect to the group of other professionals (Table 3 and Figure 3).
71% (95% CI: 210.1 to 241.9) considered the Driving Pressure (DP) level adequate for ARDS to be between 13 to 15 cm- H2O, of which 38.5% (95% CI: 72.6 to 101.4) corresponded to Kine/Pt and 31.9% (95% CI: 58.2 to 85.8) corresponded to IMS. For this variable, there was no statistically significant difference in relation to the professionals (p = 0.67) (Table 2A).
For the question: What do you consider to be the target Plateau Pressure (Pplat)? 88.2% (95% CI: 293.2 to 316.8) of professionals considered between 25 to 30 cmH2O, of which 37.1% (95% CI: 96.4 to 129.6) corresponded to Kine/Pt and 31.8% (95% CI: 80.9 to 113) to IMS. The difference between these polytomous variables (professional and Pplat) was statistically significant (p < 0.05) (Table 2A).
Associated with pulmonary artery pressure measurement, 45.4% (95% CI: 138.8 to 175.2) responded that they never use it, while 30.4% (95% CI: 88.2 to 121.8) responded that they rarely use it. The difference between these polytomous variables was statistically significant (p < 0.01) (Table 2A).

Figure 2. Probability of choice of the different variables according to the type of professional in charge.

Figure 3. Probability of choice of PEEP level according to the type of professional in charge ** All the figures described with letter A, are in the supplementary material.
Table 3. Difference in average PEEP choice levels according to profession
| Question | Mean (SD) | Coeff | p | 95% CI |
| IMS | 17.6 (4.3) | 2.5 | < 0.01 | 0.81 to 4.18 |
| IMN-S
What is the highest |
18.7 (3.7) | 3.5 | 0.06 | – 0.16 to 7.23 |
| level of PEEP you have Kine/Pt | 17.5 (3.7) | 2.4 | < 0.01 | 0.69 to 4.01 |
| used? Respiratory therapist | 15.1 (4.1) | – 0.04 | 0.98 | – 1.80 to 1.73 |
| Other | 15.1 (5.8) | – | – | – |
| IMS | 20.2 (5.1) | 1.9 | 0.10 | – 0.39 to 4.27 |
| , ,, IMN-S
What PEEP level would |
21.6 (5.9) | 3.4 | 0.23 | – 2.11 to 8.85 |
| you never exceed? Kine/Ft | 21.5 (5.6) | 3.2 | < 0.01 | 0.92 to 5.58 |
| Respiratory therapist | 18 (5.7) | – 0.2 | 0.86 | – 2.68 to 2.23 |
| Other | 18.2 (7.8) | – | – | – |
PEEP, positive end-expiratory pressure; SD, standard deviation; Coeff, mean difference.
IMS, ICU medical specialist staff; IMN-S, ICU medical non-specialist staff; Kine/Pt, kinesiologist and physiotherapist.
The results presented are based on group 5 (other professionals) as the comparator in the analysis. Values in bold are statistically significant.
Finally, the safe level of MP to minimize ergotrauma was 32.4% (95% CI: 52.8 to 79.2) for those who believe this value to be 15 J/min, of which, 31.8% (95% CI: 13.4 to 28.6) corresponded to both IMS and Rt. Likewise, 25.5% (95% CI: 39.7 to 64.3) believe that the value is 17 J/min, while 19.1% (95% CI: 27.9 to 50.1) believe that MP does not influence ergotrauma. The difference between these polytomous variables (the professionals and MP) was not statistically significant (p = 0.23) (Table 2A).
-
Discussion
This research shows an interesting international perspective on the current definition and ventilatory support for ARDS in adult patients. Through the twenty-two questions that were posed, and using a cross-sectional design, we were able to quantitatively analyze the tendency and eventually the relative risk towards certain skills and decisions on the same issues. Likewise, unlike the work of Carmichael[7]who only consulted physicians, our analysis expands the circle of health professionals who are linked to critical medicine and intensive care in a multi- and transdisciplinary manner, including professionals who deal with critical patients but outside ICU. This allowed us to provide a more global vision for the analysis of the objective of this research.
An advantage achieved through our study was to be able to generate an international qualitative-quantitative analysis with which trends can be established, according to the type of professional, towards important updated diagnostic and therapeutic concepts. It should be emphasized that these results should under no circumstances be interpreted as an official social position of health professionals working with critically ill patients.
Interestingly, our survey shows that 32.1% believe that at least three criteria are necessary to define ARDS, 11.6% of respondents believe that only one is needed, and less than 20%
believe that more than 5 criteria are necessary to define ARDS. These data coincide with those described in the initial work of Murray et al.[2], from which the recognized score is derived. Associated with this finding, the use of lung ultrasound[8]at the point of care has a great clinical approach which can replace radiography trying to optimize diagnostic times. Beyond being operator-dependent, it has had an important relevance among health professionals in ICU, although its use requires a relatively short learning curve[9].
Regarding PaO2 targets for ARDS, the ARDS Network[10]proposes values between 55 to 80 mmHg, which coincide with the results of the survey designed by Carmichael[7], while in our research 52.6% of respondents consider the minimum PaO2 to be between 86 to 90 mmHg, followed by 22.8% for 91 to 95 mmHg. This is consistent with the fact that the incorporation of international recommendations into clinical practice tends to be slow, and can take up to 17 years[11].
Daoud et al. [12], in an international survey conducted in 2021, explored the preference of clinicians regarding the preferred ventilatory mode, reporting a high heterogeneity in the preferences of the respondents. However, these findings denote that, within the conventional ventilatory modes, PC ventilation was the most common for the management of ARDS. Data that differ from those found in our investigation, where the observed results indicate that 67.3% prefer VC ventilation, while 17.3% prefer PC ventilation. It is likely that, in our survey, the preference for VC ventilation is mainly due to the multidisciplinary participation of health professionals.
For Carmichael et al.[7], the appropriate Vt for ARDS patients were between 10 to 15 ml/kg. However, Gattinoni[13]in 2005, proposed the concept called “baby lung” where, based on radiological tomographic calculations, the author determined that a diseased lung in patients with ARDS behaved like the lung of a pediatric patient between 5 and 6 years of age, proposing then to program a Vt equivalent to 6 ml/kg of ideal weight. This coincides with that reported by Carmichael et al[7]and with what was found in our work, where 56.1% of the respondents believe that the ideal Vt for these patients is between 6 to 8 ml/kg of weight, although 42.8% consider that it should be < 6 ml/kg of weight.
Related to permissive hypercapnia, Bigatello et al.[14], defined it as a ventilatory strategy for the ventilatory treatment of ARDS in those patients in whom CO2 retention is the result of hypoventilation as a consequence of low Vt management to minimize mechanical ventilator-associated lung injury (VALI), through low alveolar pressures. While Hickling et al.[15], emphasized limiting Pplat to < 30 cmH2O and allowing slow CO2 retention to levels where acute acidosis is “tolerable”. Webb et al.[16], emphasize the relevance of alveolar recruitment and not only gas exchange, taking care to avoid alveolar shear. Thus, from an integrated analysis it can be established that the safety levels for hypercapnia and acidosis persist at present in an uncertain range. According to our research, 76.9% of respondents confirm that they use this strategy, being mainly chosen by Kine/Pt. These data coincide with the results published by Carmichael[7], although they were not expressed quantitatively.
The rationale for the use of the P/F table to program PEEP in these patients is based on the clinical trial conducted by the ARDS Network group[17], where they conclude that in patients with ARDS, the programming of PEEP using this table improves gas exchange, although it did not show significant differences when mortality was evaluated. Our analysis shows that 39% of the respondents confirm the use of this tool. Kine/Pt stand out within this group.
The Lung Safe trial[18]is an international multicenter study, with prospective longitudinal design, conducted in 2014 and its main objective was to evaluate the incidence and outcomes of patients with ARDS hospitalized in intensive care units in 50 countries. The authors report on PEEP levels that 82.6% received levels below 12 cmH2O. Likewise, Hodgson et al.[19], by means of a clinical trial, compared two PEEP levels (high and low) using alveolar recruitment maneuvers by means of the technique called “staircase” (Open Lung Approach), and concluded that the intervention was associated with a lower inflammatory response (measurement of cytokines), better oxygenation and better pulmonary distensibility with respect to the control group. In a systematic review with meta-analysis of clinical trials[20], comparing the same two levels of PEEP, it is reported that there was no significant difference in mortality at 28 days of follow-up between both groups. In Carmichael’s work[7], the maximum PEEP levels did not exceed 25 cmH2O, and the mean number of respondents preferred levels between 10 and 15 cmH2O. These values agree with the results of our study: 43.3% responded that the highest PEEP levels used were between 10 to 15 cmH2O, while 49.2% responded that they never exceeded levels between 16 to 20 cmH2O.
On the other hand, 88.2% of the respondents of our investigation, consider that the target Pplat levels for ARDS patients are between 25 to 30 cmH2O, while 71.1% prefer to maintain adequate DP levels between 13 to 15 cmH2O. These values correspond to current evidence. The Lung Safe[18]mentions a mean Pplat of 24.4 cmH2O and 15.5 cmH2O of DP. Guerín et al.[21], through an analysis that included two randomized clinical trials, reported that an average DP of 13.5 cmH2O was associated with an increased risk of mortality in patients with ARDS.
Likewise, associated with the use of pulmonary artery wedge pressure measurement, current definitions have eliminated this parameter due to the high risk of its installation and the poor benefit it shows in these patients. In our results, the responses show that 45.4% never use this procedure, and only 30.4% use it rarely. Carmichael[7]on the other hand, reported that levels < 15 cmH2O were related to safe PEEP levels.
Currently, many authors have attempted to redefine ARDS. Many of the new proposals include both high-flow nasal cannula oxygen therapy (HFNC) and S/F ratio in them. Yuan et al.[22], propose a S/F < 315 mmHg as a value equivalent to a P/F < 300 mmHg and further mention that other authors[23]propose a requirement of at least 30 L/min of HFNC as diagnostic criteria for ARDS. A similar and more recent approach is that proposed by Van der Ven et al.[24], for ARDS by COVID 19 , who assume that 30 L/min HFNC is capable of providing 5 cmH2O of PEEP and could replace the classic definition that includes only PEEP from the invasive mechanical ventilator. However, for 73.1% of the respondents in our investigation, there was agreement to include the S/F parameter in the definition of ARDS and 50.3% were not in favor of including HFNC in this definition.
The measurement of energy transfer to quantify the risk of ergotrauma has been simplified using the MP[25]formula. Until now the use of this variable has been solely in the context of minimizing VALI[26]. However, some works have tried to use this variable within the definition of ARDS. Costa et al.[27], reported in their meta-analysis how MP was associated with higher mortality with values > 15 J/min, values similar to those obtained by Haudebourg et al.[28], through observational studies. Likewise, our survey results show that 32.4% of respondents believe that 15 J/min is a safe value to minimize ergotrauma, while 59.3% of respondents stated not to use this variable.
Finally, multidisciplinary work is considered the fundamental and complementary base for the success of critical patient care. The differences found in this survey with respect to the different professionals are probably due to the heterogeneity in the conformation and management of critical care units worldwide.
-
Conclusions
The intervention guidelines and practice patterns reported and analyzed by the respondents in this research, associated with the diagnosis and treatment from ventilatory strategies in patients with ARDS, differ widely among the different health professionals in critical care, and even within professionals of the same group.
Author’s contributions : AF-C: Writing original draft, conceptualization and visualization, formal analysis, methodology, design and interpretation. FP: Writing, data collection, reviewing and editing, conceptualization, visualization. CC-M: Writing, data collection, validation, reviewing and editing. EM-Z: original idea, conceptualization, visualization. YL-F, MI-E, AR-R, AG-C, AM, VM-A, AM-R: Data recollection, reviewing and validation.
Acknowledgements : We would like to express our most sincere thanks to all those who, in some way, collaborated in the realization, promotion and dissemination of this survey. Mainly to: Rodrigo Adasme-Jeria, William Cristancho, Ehab Daoud, Mariano Chávez, Noel Díaz, Oscar Cabrera, Natalia Caballero, MartínManagó. And to the following Societies: Asociación Salvadoreña de Medicina Crítica y Cuidados Intensivos (ASALMECCI), Sociedad Latinoamericana de Cuidados Respiratorios (SOLACUR), SOCIETY OF MECHANICAL VENTILATION (SMV), Federación Latinoamericana de Enfermería en Cuidados Intensivos (FLECI), and Asociación Colombiana de Medicina Crítica y Cuidado Intensivo (AMCI) for their active collaboration in the dissemination of the survey.
Running head: An international survey-based study quantifying the opinions of health care professionals regarding the diagnosis and up-to-date management of ARDS, compared with similar work by Carmichael et al. in 1996.
Conflict of Interest: All authors declare no conflict of interest.
Funding: This work did not receive any funding.
-
References
1. Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967 Aug;2(7511):319–23. https://doi.org/10.1016/S0140-6736(67)90168-7 PMID:4143721
2. Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988 Sep;138(3):720–3. https://doi.org/10.1164/ajrccm/138.3.720 PMID:3202424
3. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818–24. https://doi.org/10.1164/ajrccm.149.3.7509706 PMID:7509706
4. Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012 Oct;38(10):1573–82. https://doi.org/10.1007/s00134-012-2682-1 PMID:22926653
5. Grasselli G, Calfee CS, Camporota L, Poole D, Amato MB, Antonelli M, et al.; European Society of Intensive Care Medicine Taskforce on ARDS. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023 Jul;49(7):727–59. https://doi.org/10.1007/s00134-023-07050-7 PMID:37326646
6. Matthay MA, Arabi Y, Arroliga AC, Bernard G, Bersten AD, Brochard LJ, et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2023 Jul. PMID:37487152
7. Carmichael LC, Dorinsky PM, Higgins SB, Bernard GR, Dupont WD, Swindell B, et al. Diagnosis and therapy of acute respiratory distress syndrome in adults: an international survey. J Crit Care. 1996 Mar;11(1):9–18. https://doi.org/10.1016/S0883-9441(96)90015-5 PMID:8904279
8. Costamagna A, Pivetta E, Goffi A, Steinberg I, Arina P, Mazzeo AT, et al. Clinical performance of lung ultrasound in predicting ARDS morphology [Internet]. Ann Intensive Care. 2021 Mar;11(1):51. https://doi.org/10.1186/s13613-021-00837-1 PMID:33779834
9. Breunig M, Hanson A, Huckabee M. Learning curves for point-of-care ultrasound image acquisition for novice learners in a longitudinal curriculum. ultrasound J. 2023 Jul;15(1):31.
10. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N Engl J Med. 2000 May;342(18):1301–8. https://doi.org/10.1056/NEJM200005043421801 PMID:10793162
11. Ibarra-Estrada M, Veith J, Mireles-Cabodevila E. Implementing change is a science [Internet]. Med Intensiva (Engl Ed). 2022 Jul;46(7):359–62. Available from: https://www.medintensiva.org/es-implementing-change-is-science-articulo-S0210569122001474 https://doi.org/10.1016/j.medine.2022.05.011 PMID:35753709
12. daoud, Yamasaki K, Sanderson R, Shokry M. daoud ehab, Yamasaki K, Sanderson R, Shokry M. Mechanical ventilation modes utilization. An international survey of clinicians. J Mech Vent. 2021;2(3):105–11. https://doi.org/10.53097/JMV.10031.
13. Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005 Jun;31(6):776–84. https://doi.org/10.1007/s00134-005-2627-z PMID:15812622
14. Bigatello LM, Patroniti N, Sangalli F. Permissive hypercapnia. Curr Opin Crit Care. 2001 Feb;7(1):34–40. https://doi.org/10.1097/00075198-200102000-00006 PMID:11373509
15. Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16(6):372–7. https://doi.org/10.1007/BF01735174 PMID:2246418
16. Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974 Nov;110(5):556–65. PMID:4611290
17. Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al.; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004 Jul;351(4):327–36. https://doi.org/10.1056/NEJMoa032193 PMID:15269312
18. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al.; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016 Feb;315(8):788–800. https://doi.org/10.1001/jama.2016.0291 PMID:26903337
19. Hodgson CL, Tuxen DV, Davies AR, Bailey MJ, Higgins AM, Holland AE, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011;15(3):R133. https://doi.org/10.1186/cc10249 PMID:21635753
20. Liang M, Chen X. Differential Prognostic Analysis of Higher and Lower PEEP in ARDS Patients: Systematic Review and Meta-Analysis. J Healthc Eng. 2022 Mar;2022:5399416. https://doi.org/10.1155/2022/5399416 PMID:35356616
21. Guérin C, Papazian L, Reignier J, Ayzac L, Loundou A, Forel JM; investigators of the Acurasys and Proseva trials. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials [Internet]. Crit Care. 2016 Nov;20(1):384. https://doi.org/10.1186/s13054-016-1556-2 PMID:27894328
22. Yuan X, Pan C, Xie J, Qiu H, Liu L. An expanded definition of acute respiratory distress syndrome: challenging the status quo [Internet]. J Intensive Med. 2022 Jul;3(1):62–4. Available from: https://www.sciencedirect.com/science/article/pii/S2667100X22000664 https://doi.org/10.1016/j.jointm.2022.06.002 PMID:36785583
23. Matthay MA, Thompson BT, Ware LB. The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? [Internet]. Lancet Respir Med. 2021 Aug;9(8):933–6. https://doi.org/10.1016/S2213-2600(21)00105-3 PMID:33915103
24. van der Ven FL, Valk CM, Blok S, Brouwer MG, Go DM, Lokhorst A, et al.; PRoAcT–COVID study investigators. Broadening the Berlin definition of ARDS to patients receiving high-flow nasal oxygen: an observational study in patients with acute hypoxemic respiratory failure due to COVID-19. Ann Intensive Care. 2023 Jul;13(1):64. https://doi.org/10.1186/s13613-023-01161-6 PMID:37452196
25. Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016 Oct;42(10):1567–75. https://doi.org/10.1007/s00134-016-4505-2 PMID:27620287
26. Wu HP, Leu SW, Lin SW, Hung CY, Chen NH, Hu HC, et al. Role of Changes in Driving Pressure and Mechanical Power in Predicting Mortality in Patients with Acute Respiratory Distress Syndrome [Internet]. Diagnostics (Basel). 2023 Mar;13(7):1226. Available from: https://www.mdpi.com/2075-4418/13/7/1226 https://doi.org/10.3390/diagnostics13071226 PMID:37046444
27. Costa EL, Slutsky AS, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, et al. Ventilatory Variables and Mechanical Power in Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2021 Aug;204(3):303–11. https://doi.org/10.1164/rccm.202009-3467OC PMID:33784486
28. Haudebourg AF, Tuffet S, Perier F, Razazi K, de Prost N, Mekontso Dessap A, et al. Driving pressure-guided ventilation decreases the mechanical power compared to predicted body weight-guided ventilation in the Acute Respiratory Distress Syndrome. Crit Care. 2022 Jun;26(1):185. https://doi.org/10.1186/s13054-022-04054-5 PMID:35725498

 ORCID
ORCID