Leonado Arce Gálvez1,2,* Alejandra Ceballos Vejarano2,4, Lina María Rodríguez Vélez1, Diana Alejandra Méndez Vega3
Recibido: 15-08-2024
Aceptado: 12-12-2024
©2025 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 54 Núm. 2 pp. 142-144|https://doi.org/10.25237/revchilanestv54n2-07
PDF|ePub|RIS
Opinión actual y condiciones de seguridad
Abstract
Ketamine is a widely used drug in anesthesiology, it has different pharmacological action points including glutamatergic and non-glutamatergic receptors with interesting potential in the treatment of different pain conditions refractory to conventional measures. In the context of opioid dependence, it is a useful tool in the control of pain symptoms. Its use in pregnant patients has been cautious over time, but there are more and more studies showing its safety profile, especially after the first trimester of gestation. The analgesic doses of this drug are lower than those recorded in the management of any other condition, which presents a safe and fascinating alternative for management during pregnancy; however, more studies are required to be able to draw generalized conclusions.
Resumen
La ketamina es un fármaco ampliamente utilizado en anestesiología, tiene diferentes puntos de acción farmacológica incluyendo receptores glutamatérgicos y no glutamatérgicos con interesante potencial en el tratamiento de diferentes cuadros de dolor refractarios a las medidas convencionales. En el contexto de la dependencia de opiáceos, es una herramienta útil en el control de los síntomas del dolor. Su uso en pacientes embarazadas ha sido cauteloso a lo largo del tiempo, pero cada vez hay más estudios que demuestran su perfil de seguridad, especialmente a partir del primer trimestre de gestación. Las dosis analgésicas de este fármaco son inferiores a las registradas en el manejo de cualquier otra patología, lo que presenta una alternativa segura e interesante para el manejo durante el embarazo; sin embargo, se requieren más estudios para poder extraer conclusiones generalizadas.
-
Introduction
Ketamine is an analgesic drug that has been available for more than 60 years[1]. However, its analgesic potential has continued to develop in more aspects due to its pharmacological characteristics with a potential effect on patients with chronic oncologic and non-oncologic pain in the face of the global opioid crisis that has become known[2].
On the other hand, the management of pain in the pregnant patient is a challenge, given that not only is the safety and dependence profile of the future mother important, but also the potential risks to which the fetus may be exposed with medications such as anti-inflammatory and opioid drugs[3]. The first line of management in pregnant women is therefore based on non-pharmacological measures with physical therapy, psychological interventions, or interventional management; however, there are clinical conditions in which the use of systemic analgesics is imperative and safe interventions should be sought[4].
Ketamine does not have a safety classification assigned by the Food and Drug Administration (FDA) of the United States, but it is classified as B3 in gestation by the Therapeutic Goods Administration (TGA) of Australia, which means that it has been used in groups of pregnant women without finding damage to the fetuses or risk of malformations, with animal studies showing some fetal damage without being able to be demonstrated in humans[4]. The use of ketamine has been increasing in pregnant patients with concomitant psychiatric conditions with adequate maternal-fetal outcomes, which provides safety information that can be used for the use of this drug in pregnant women with pain conditions refractory to conventional management during pregnancy.
-
Pharmacology
From the pharmacological point of view, ketamine is a water-soluble drug with a pH between 3.5 and 5.5 with a dissociation constant (pKa) of 7.5; it is found as a racemic mixture of two enantiomers the S(+) ketamine which is up to 4 times more potent than its isomer R(-) ketamine and generates a hypnotic effect 1.5 times more potent[1]. It is metabolized in the liver by cytochrome p450 in the company of different microsomal enzymes, however, this process is still not entirely clear, finding that ketamine has the potential to inhibit some cytochromes belonging to p450 which explains its tachyphylaxis[5]. It binds 10-30% to protein, so it rapidly reaches deep tissues such as the brain, heart, and lungs. Its main active metabolite is norketamine at 80% but also hydroxynorketamine at 15% and hydroxyketamine at 5% [6]. Its elimination half-life is 2-3 hours in a tricompartmental model where renal clearance is higher in men than in women which is a important point in the pregnant patient[5].
Norketamine is an active metabolite is present in blood 2 minutes after the application of intravenous ketamine, with a peak at 30 minutes. Most ketamine and its metabolites are excreted in urine, however, an alteration in renal function could only eliminate 20% of this drug compared to patients with normal renal function[5],[6].
The classic mechanism of action of ketamine is the inhibition of NMDA (N-Methyl-D-Aspartate) glutamatergic receptors in the central nervous system, which generates its analgesic, amnesic, psychosensory, and neuroprotective action. However, other mechanisms of action not measured by glutamatergic receptors have been described[7]. Among these mechanisms of action are serotonin receptor inhibition, activation of noradrenergic pathways and inhibition of catecholamine reuptake (noradrenaline and dopamine), antagonism of dopaminergic receptors, potentiates stimulation of inhibitory GABA-A receptors, binds to opioid receptors Mu, Delta, and Kappa receptors (without generating a direct opioid analgesic action but occupying the receptor and being able through Kappa receptors to generate psychotic effect), cholinergic potential in the hippocampus with the stimulation of muscarinic and nicotinic receptors, and finally has action on ion channels; sodium channels in a similar way to local anesthetics and also to a lesser extent on potassium channels and L-type calcium channels[5]-[7] (Figure 1).
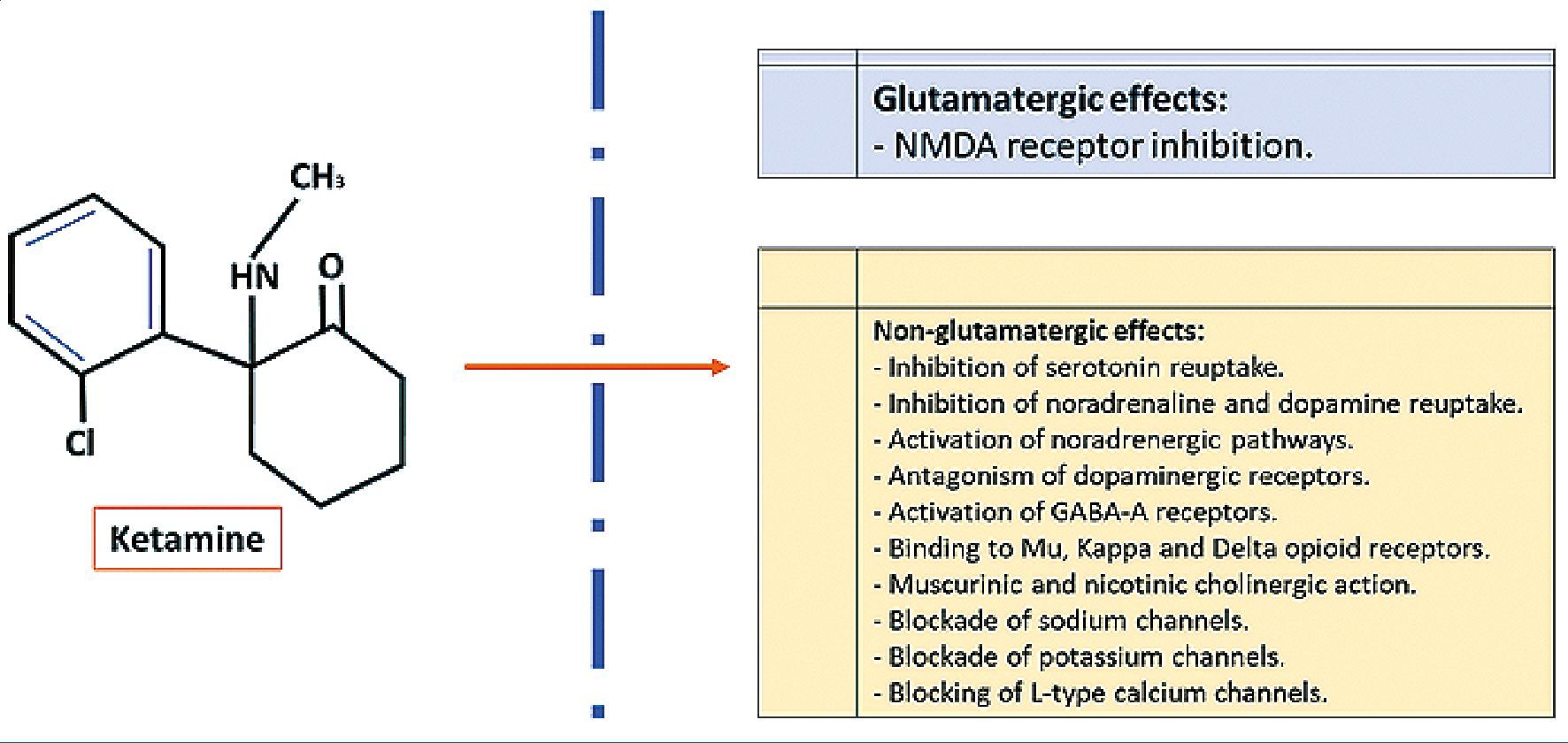
Figure 1. Mechanism of action of ketamine.
-
Ketamine and gestation
The multiple pharmacological routes of action of ketamine gives it a role in the control of refractory chronic pain, however, its chemical characteristics place it in a short-term intervention that could be safely used in pregnant patients. In the analgesic use of ketamine, the dose is one of the most important safety points, in the use of anesthetic induction the usual doses are higher than 1 mg (milligram) per kg/h (kilogram/hour), for pain control doses ranging from 0.1 mg/kg/h are usually used, which are titrated according to the patient’s response up to a maximum of 0.5 mg/kg/h[8]. Studies of the placental passage of ketamine has been performed where the concentrations of micrograms of ketamine are very similar between maternal and newborn plasma, but inadequate perinatal outcomes were not generated; Even though there were ketamine doses higher than 2 mg/kg/h, which is 4 times the maximum dose used in analgesia[9].
In the treatment of depressive disorders, ketamine has been used at doses of 0.5mg/kg/h and higher with adequate safety outcomes for mother and newborn, without presenting any type of perinatal complication[10] and extrapolating these safety conclusions to analgesic management where lower doses are used, it could become an interesting intervention[11]. In animal models different doses of ketamine have been used, finding that the effects on fetal neurodevelopment depend on the dose administered, finding a delay in neurodevelopment and a compromise in synaptic formation, but only at high dos- es[12]; On the other hand, it has been demonstrated in these animal models that in conditions of stress or inflammation ketamine has a neuroprotective effect on the newborn [13], which is interesting in conditions of comorbidity or concomitant use of other analgesics including situations of opioid abuse, which have shown perinatal effects with a higher percentage of fetal complications, low birth weight, low height for gestational age and risk of neonatal abstinence[14].
-
Conclusion
Ketamine has a wide range of pharmacological action with an important potential in the management of refractory pain conditions, due to its biochemical characteristics it is used for short periods of time, and unlike its uses in anesthesiology and psychiatry, the analgesic concentrations are very low. Due to the findings in animal studies, its use should be restricted in the first trimester of gestation trying to avoid any possibility of alteration in neurodevelopment, but at other times of gestation its use has a good safety profile supported by adequate perinatal outcomes at higher doses, and it can also be a protective factor against stressful or inflammatory conditions that generate pain and also be an alternative to opioid use that have a proven negative impact on the mother-child binomial. More studies in humans are clearly required to make clear decisions or draw additional conclusions, but with this review, we have an interesting alternative in the management of pain in our current pregnant population.
Funding: The authors did not receive funding to carry out this study.
Conflict of interest: We declare no conflict of interest.
-
References
1. López-Millán JM, Sánchez-Blanco C. Utilización de ketamina en el tratamiento del dolor agudo y crónico. n.d.
2. Strathdee SA, Goodman-Meza D, Rafful CM. Addressing opioid use disorder: mexico’s step backwards. Lancet Reg Health Am. 2023 May;23:100520. https://doi.org/10.1016/j.lana.2023.100520 PMID:37497392
3. Ray-Griffith SL, Wendel MP, Stowe ZN, Magann EF. Chronic pain during pregnancy: a review of the literature. Int J Womens Health. 2018 Apr;10:153–64. https://doi.org/10.2147/IJWH.S151845 PMID:29692634
4. Arce-Gálvez L, Méndez-Vega DA, Mancera-Álzate JM, Benavídez-Ramírez A, Rodríguez-Vélez LM. Low back pain in pregnancy, pathophysiological aspects and treatment. Rev Chil Obstet Ginecol. 2022;87:111–21. https://doi.org/10.24875/RECHOG.22000004.
5. Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther. 2013 Jun;19(6):370–80. https://doi.org/10.1111/cns.12099 PMID:23575437
6. Gales A, Maxwell S. Ketamine: Recent Evidence and Current Uses. 2018.
7. Henthorn TK, Krejcie TC, Niemann CU, Enders-Klein C, Shanks CA, Avram MJ. Ketamine Distribution Described by a Recirculatory Pharmacokinetic Model Is Not Stereoselective. Volume 91. 1999.
8. Vadivelu N, Schermer E, Kodumudi V, Belani K, Urman RD, Kaye AD. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298–306. https://doi.org/10.4103/0970-9185.168149 PMID:27625475
9. Ellingson A, Haram K, Solheim E. Transplacental Passage of Ketamine after Intravenous Administration. Volume 21. 1977.
10. Patel A, Saucier AC, Hobday C, Chacko R. Safety and efficacy of ketamine-augmented electroconvulsive therapy in third trimester pregnancy complicated by COVID-19. Proc Bayl Univ Med Cent. 2022 Aug;35(6):874–5. https://doi.org/10.1080/08998280.2022.2106415 PMID:36304628
11. Alipoor M, Loripoor M, Kazemi M, Farahbakhsh F, Sarkoohi A. The effect of ketamine on preventing postpartum depression. J Med Life. 2021;14(1):87–92. https://doi.org/10.25122/jml-2020-0116 PMID:33767791
12. Dong C, Rovnaghi CR, Anand KJ. Ketamine exposure during embryogenesis inhibits cellular proliferation in rat fetal cortical neurogenic regions. Acta Anaesthesiol Scand. 2016 May;60(5):579–87. https://doi.org/10.1111/aas.12689 PMID:26822861
13. Cheung HM, Yew DT. Effects of perinatal exposure to ketamine on the developing brain. Front Neurosci. 2019 Feb;13:138. https://doi.org/10.3389/fnins.2019.00138 PMID:30853884
14. Harter K. Opioid use disorder in pregnancy. Ment Health Clin. 2019 Nov;9(6):359–72. https://doi.org/10.9740/mhc.2019.11.359 PMID:31857932

 ORCID
ORCID