Daniel Pérez-Ajami MD.1,*, Ignacio Albero MD.2, Iratxe Zarragoikoetxea MD.2, Elisa Viscasillas Navarro MD.3, Pilar Argente Navarro MD., PhD.4
Recibido: 14-05-2023
Aceptado: 28-05-2023
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 6 pp. 623-625|https://doi.org/10.25237/revchilanestv52n6-11
PDF|ePub|RIS
Trombo moldeado de la cánula de ECMO en la vena cava inferior
Abstract
A 21-year-old male patient who was admitted in the ICU after multiple traumas suffered hemodynamic and respiratory deterioration requiring peripheral veno-venous extracorporeal membrane oxygenation (VV ECMO) support as a bridge to recovery within the first forty-eight hours of his hospital admission. Thanks to this therapy, he significantly improved allowing the withdrawal of ECMO 144 hours after its implantation. However, the bedside ultrasound exam performed after the ECMO removal reported a hyperechogenic mass inside in the inferior vena cava (IVC). Due to its sonographic characteristics, a molding thrombus of the femoral ECMO cannula was suspected. Subcutaneous enoxaparin was given for 1 mg/ kg every 12 hours until the thrombus disappeared.
Resumen
Paciente varón de 21 años que ingresa en la UCI tras un politraumatismo con TCE severo que sufre un deterioro rápidamente progresivo tanto hemodinámico como respiratorio que requirió de soporte tipo ECMO veno-venosa periférico como puente a la recuperación en las primeras cuarenta y ocho horas de su admisión hospitalaria. Gracias a esta terapia, su estado mejoró notablemente permitiendo la retirada del ECMO a las 144 h de su implantación. Sin embargo, en el examen ecográfico realizado a pie de cama en la UCI tras la extracción del dispositivo se objetivó una masa hiperecogénica en el interior de la vena cava inferior (VCI). Por sus características ecográficas se sospechó un trombo moldeando la cánula femoral del ECMO. Como tratamiento se administró de 1 mg/kg cada 12 h de enoxaparina subcutánea con seguimiento ecográfico hasta la desaparición del trombo.
-
Case report
A21-year-old male patient was admitted to a tertiary hospital Intensive Care Unit (ICU) because he suffered multiple traumas after a seven-meter-high drop. Upon arrival, he was conscious, hemodynamically stable and with correct gas exchange. The total body CT scan revealed an unstable fracture of the right posterior arch of the pelvis causing an extensive retroperitoneal hematoma with active pelvic bleeding and pulmonary consolidation in the lower lobes of both lungs. The cranial CT scan reported a traumatic brain injury (TBI) with multiple cerebral concussions and a mild subarachnoid hemorrhage.
Twenty-four hours following his admission, the patient suffered hemodynamic deterioration requiring norepinephrine up to 1 microgram/kg/min and vasopressin up to 1,8 IU/h. Concomitantly, he developed an acute pulmonary distress (ARDS) due to thoracic trauma, requiring orotracheal intubation with lung protective ventilation, muscle relaxation and inhaled nitric oxide up to 20 ppm. Because of external fixation of the pelvis, the prone position was formally contraindicated. Twelve hours later, the regular treatment was unsuccessful, and his condition was still critical with PO2/FiO2 < 80. Normal biventricular function was observed in a transthoracic echocardiography and therefore right femoro-jugular VV ECMO was implanted as the last line of treatment. Due to both pelvis fracture and traumatic brain injury, he did not receive anticoagulation during the first forty-eight hours, but the flow rate was established above 3,5 liters per minute to avoid thrombosis. When the pelvic hemorrhage was controlled by embolization of branches of the internal iliac artery, and the second cranial CT reported stabilization of brain injuries after 48 hours, intravenous unfractionated heparin was started to maintain APTT of between 40-50 seconds and ACT between 160-180 seconds. He presented a significant improvement allowing ECMO removal six days after its implantation. The third CT scan, which was performed after ECMO removal, did not report any new cerebral lesions or an increase in existing ones.
However, after the ECMO withdrawal, a hyperechogenic image was observed in the bedside ultrasound in the IVC extending from the right atrium to the left renal vein ostium representing the mold of the ECMO femoral cannula. Due to its sonographic characteristics, the size, and the location, a molding thrombus of the femoral cannula was suspected (Figure 1).
Subcutaneous enoxaparin was given for 1 mg/kg every 12 hours until the thrombus disappeared after fifteen days of therapy (Figure 2). During the treatment, anti-Xa activity test was monitored with a target range between 0.5-1 IU/ml. After the disappearance of the thrombus, the patient continued anticoagulant treatment during his stay on the hospital ward.
-
Discussion
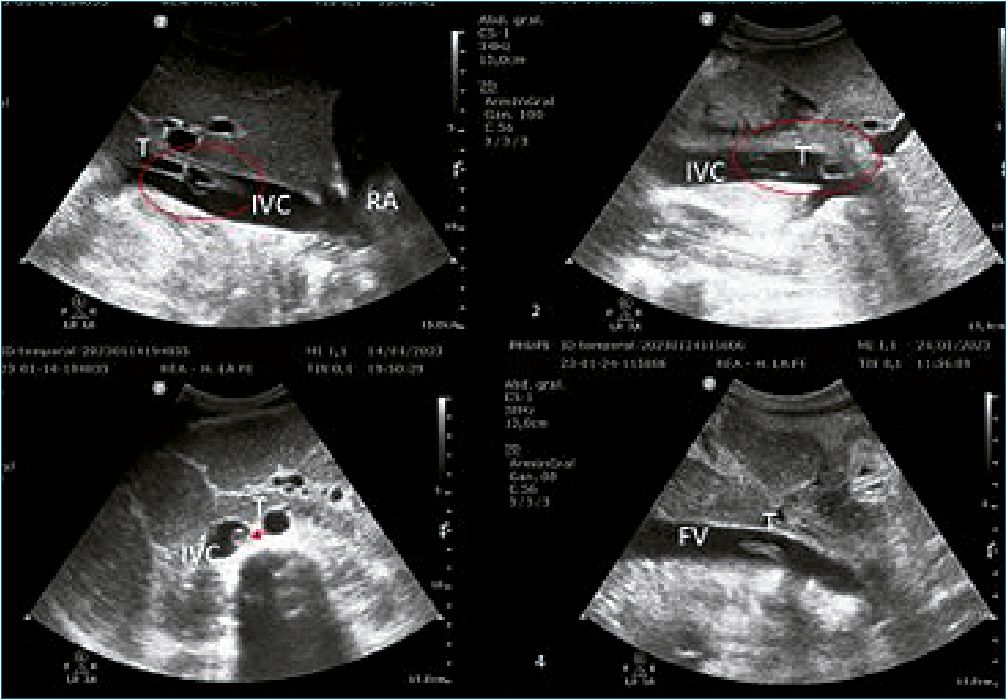
Figure 1. Echocardiography after ECMO removal. 1: Inferior vena cava thrombosis close to the right atrium; 2: The thrombus inside the inferior vena cava in the liver area; 3: The thrombus just after the union of the iliac veins; 4: The thrombus inside the femoral vein. (IVC: inferior vena cava; RA: right atrium; T: thrombus; FV: femoral vein).
VV ECMO is known to be the last line treatment in ARDS with controversial results in randomized controlled trials[1],[2] and with high rate of morbimortality due to its invasiveness and risk of bleeding.
The prevalence of ARDS in polytraumatism remains around 13%-18%[3] and its mortality can be as high as 50%-80%[3]. ECMO in ARDS provides blood oxygenation and CO2 removal allowing to perform a protective ventilation strategy and providing hemodynamic stability. Despite the development of more biocompatible surfaces such as polymethylpentene membranes and the use of phosphorylcholine-coated tubing, the use of ECMO implies anticoagulation therapy. Though hypoxemia is one of the main priorities to avoid in a TBI traumatic brain injury since it has been identified as a secondary injury to TBI itself associated with a poor prognosis[4]. Hence, in these cases of young patients with TBI, ECMO may help prevent hypoxemia and its consequences[4].
In this case, we ran VV ECMO without anticoagulation during the first 48 hours because of the life-threatening pelvic bleeding and TBI. To prevent thrombotic events, the use of high ECMO flows have been described if heparin cannot be administrated[5],[6], which must be initiated as soon as the patient’s condition allows it considering the potential bleeding risk. Heparin administration was delayed until the CT scan informed stability of the brain injuries although the exact time to ensure stability is not well established. Thus, it is mandatory to discuss and consider all the options and make a risk-benefit balance of b therapy or delaying it, depending on the severity and location of the intracranial injuries and the presence or absence of thrombus in the ECMO oxygenator.
Nonetheless, thrombotic events during ECMO are also relatively frequent, reaching up to 10%[7]. They are generally of subclinical characteristics such as thrombi in the oxygenator that do not affect the patient but require continuous surveillance and monitoring due to their potential risks such as loss of device function[8]. The polytrauma and TBI patients may receive insufficient anticoagulation due to the bleeding risk, which is also an important reason for thrombosis. Moreover, a thrombus in the IVC can lead to massive pulmonary thrombo- embolism[9] or paradoxical embolism if there is an intracardiac shunt which was discarded in this case. It is important to know that clinical manifestations of IVC thrombosis in ECMO patients are usually nonspecific like distal limb edema, self-limited minutes of hypoxemia or ECMO flow decrease[9] so the physicians must have a high index of suspicion and it is recommended to perform an ultrasound exam daily while the ECMO is running or more exams if the patient conditions undergo an acute change[6],[10],[11]. Furthermore, a protocolized practice in our center is that even if no thrombus is observed through the US exam, long term anticoagulation regime can be recommended due to the high risk of thrombosis because of endothelial damage.
Although anticoagulation was the only treatment the patient received, another option to consider is percutaneous extraction using the AngioVac® thrombus aspiration proce- dure[12]. The complication rate of this technique is low but rupture of the cardiac chambers or injury to the tricuspid valve are life-threatening. The main limitations are its high cost, the risk of complications and the exiguous current scientific evi- dence[12]. For all these reasons, we consider that this treatment should be considered as an alternative to medical or surgical treatment in cases in which anticoagulant therapy is not possible.
To sum up, the use of ECMO in polytrauma patients is a controversial issue due to the presence of intracranial and massive bleeding lesions and the need for anticoagulation. On the other hand, they are generally young patients with no comorbidities and long-life expectancy if the acute process is resolved. Consequently, ECMO implantation even with cranial bleeding lesions has been reported with encouraging results[7]. Even though some tips must be considered: high ECMO flow rate, patient and ECMO configuration selection, CT scan monitoring, heparin introductions as soon as possible and long-term heparin therapy and systematic sonographic exam should be performed.
To conclude, ECMO support is a highly specialized therapy that implies a multidisciplinary process and high level of experience is cornerstone in the context of polytraumatism.
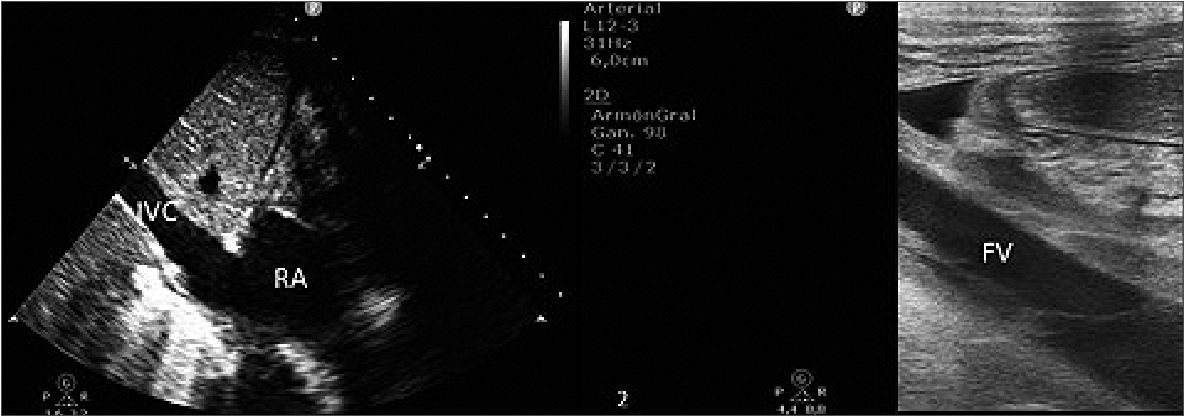
Figure 2. Echocardiography after the anticoagulant treatment with subcutaneous low-molecular-weight heparin. The disappearance of the thrombus in the inferior vena cava (1) and femoral vein; (2) is observed. IVC: inferior vena cava, RA: right atrium, FV: femoral vein).
Referencies
1. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al.; EOLIA Trial Group, REVA, and ECMONet. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018 May;378(21):1965–75. https://doi.org/10.1056/NEJMoa1800385 PMID:29791822
2. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al.; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009 Oct;374(9698):1351–63. https://doi.org/10.1016/S0140-6736(09)61069-2 PMID:19762075
3. van Wessem KJ, Leenen LP. Incidence of acute respiratory distress syndrome and associated mortality in a polytrauma population. Trauma Surg Acute Care Open. 2018 Dec;3(1):e000232. https://doi.org/10.1136/tsaco-2018-000232 PMID:30623025
4. Contreras, Mario & Barrera, Sihara & Orozco, Héctor & Quintana Pájaro, Loraine & Corrales Santander, Hugo & Moscote-Salazar, Luis. (2018). Ventilación mecánica en el paciente con trauma cerebral Mechanical ventilation in patients affected by traumatic brain injury.
5. Zarragoikoetxea I, Pajares A, Moreno I, Porta J, Koller T, Cegarra V, et al. Documento de consenso SEDAR/SECCE sobre el manejo de ECMO. Rev Esp Anestesiol Reanim. 2021;68(8):443–71. https://doi.org/10.1016/j.redar.2020.12.011.
6. Lamb KM, Hirose H. Vascular Complications in Extracoporeal Membrane Oxygenation. Crit Care Clin. 2017 Oct;33(4):813–24. https://doi.org/10.1016/j.ccc.2017.06.004 PMID:28887929
7. Vaquer S, de Haro C, Peruga P, Oliva JC, Artigas A. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care. 2017 Dec;7(1):51. https://doi.org/10.1186/s13613-017-0275-4 PMID:28500585
8. Chen Y, Chen H, Liu X, Hong C, Zhang H. Inferior vena cava thrombosis during extracorporeal membrane oxygenation: a case report and review of the literature. J Med Case Rep. 2021 Oct;15(1):529. https://doi.org/10.1186/s13256-021-03057-0 PMID:34663446
9. Bein T, Philipp A, Pregler B, Noeva E, Graf BM, Stroszczynski C. Long-segment caval thrombus after removal of ECMO cannula. Intensive Care Med. 2015 Nov;41(11):1967–8. https://doi.org/10.1007/s00134-015-3781-6 PMID:25860445
10. Platts DG, Sedgwick JF, Burstow DJ, Mullany DV, Fraser JF. The role of echocardiography in the management of patients supported by extracorporeal membrane oxygenation. J Am Soc Echocardiogr. 2012 Feb;25(2):131–41. https://doi.org/10.1016/j.echo.2011.11.009 PMID:22169046
11. Morimont P, Lambermont B, Gaspard V, Defraigne JO. Molding thrombus of an ECMO cannula floating in the right atrium. Intensive Care Med. 2015 Nov;41(11):1965–6. https://doi.org/10.1007/s00134-015-3779-0 PMID:25851390
12. Todoran TM, Sobieszczyk PS, Levy MS, Perry TE, Shook DC, Kinlay S, et al. Percutaneous extraction of right atrial mass using the Angiovac aspiration system. J Vasc Interv Radiol. 2011 Sep;22(9):1345–7. https://doi.org/10.1016/j.jvir.2011.04.004 PMID:21856509

 ORCID
ORCID