Ana M. Suárez1,*, Nathalia J. Gamarra2, Andrés F. Zuluaga1, Juan F. Parada-Márquez1
Recibido: 15-05-2024
Aceptado: 13-08-2024
©2025 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 54 Núm. 1 pp. 38-45|https://doi.org/10.25237/revchilanestv54n1-04
PDF|ePub|RIS
Abstract
Rebound pain, which lacks standardized measurements, limits the understanding of its incidence. We investigated this using various peripheral nerve blocks and explored preventative strategies. A literature search from October to December 2023 in databases such as EMBASE and PubMed yielded 208 studies, 20 of which met the inclusion criteria. The incidence of rebound pain varied widely (6.67% to 81%). Preventive approaches include perineural/intravenous dexamethasone, ketamine, continuous perineural infusion, liposomal bupivacaine, and transauricular vagus nerve stimulation. Intravenous dexamethasone consistently resulted in a significant reduction. This review highlights the high incidence of rebound pain in patients with peripheral nerve block, prompting the exploration of diverse preventative methods, with intravenous dexamethasone emerging as pivotal.
Resumen
El dolor de rebote, que carece de medidas estandarizadas, limita la comprensión de su incidencia. Investigamos esto utilizando varios bloqueos nerviosos periféricos y exploramos estrategias preventivas. Una búsqueda de literatura de octubre a diciembre de 2023 en bases de datos como EMBASE y PubMed arrojó 208 estudios, de los cuales 20 cumplieron con los criterios de inclusión. La incidencia de dolor de rebote varió ampliamente (6,67% a 81%). Los enfoques preventivos incluyen dexametasona perineural/intravenosa, ketamina, infusión perineural continua, bupivacaína liposomal y estimulación del nervio vago transauricular. La dexametasona intravenosa resultó consistentemente en una reducción significativa del mismo. Esta revisión destaca la alta incidencia de dolor de rebote en pacientes con bloqueo nervioso periférico, lo que impulsa la exploración de diversos métodos preventivos, con la dexametasona intravenosa emergiendo como clave.
-
Introduction
Regional anesthesia as a multimodal analgesia strategy is a useful tool for the management of peri- and postoperative pain[1]. Peripheral nerve blockade reduces the need for opioid use, minimizes postoperative nausea and vomiting, and reduces the length of the hospital stay[2]. Despite its effectiveness, it has been associated with rebound pain. Rebound pain has been described as a sudden and severe increase in pain, previously well-controlled, that temporally coincides with the resolution of the peripheral block, generally 8-24 hours after its administration[1]. Despite the transient nature of rebound pain, it negatively impacts patient satisfaction and can offset the benefits of regional anesthesia, leading to increased consumption of opioids and other analgesics.
Currently, there is no standardized measure for evaluating rebound pain. Williams et al defined it as the highest pain score reported by the patient in the first 12 hours after the block was not producing relief minus the score reported by the patient the last time the block was producing relief[3]. Other researchers have defined a threshold criterion, designating a score exceeding 6 in a home setting after categorizing pain intensity as mild within the post-anesthesia recovery unit[4].
Multiple approaches have been suggested to mitigate rebound pain. Among the strategies described, it is proposed to prolong the effect of the nerve block beyond the point of nociception produced by the surgical stimulus with adjuvants or the implementation of continuous blocks[2]. Continuous blocks constitute a good analgesic alternative; however, they require greater expertise and more resources, and are associated with an increased risk of complications at the block site, as well as perineural catheter failure. To date, no standardized practice has been established to prevent rebound pain[2].
Rebound pain limits the analgesic benefits of regional anesthesia. Owing to the absence of a standardized measure to evaluate this entity, information regarding its incidence is limited. However, there is no consensus to date on strategies to prevent and improve rebound pain; the available literature is limited and heterogeneous. Furthermore, owing to the diversity of blockages and contexts of use, it is difficult to establish a homogeneous practice for its prevention. Additionally, given its deleterious effects on patient satisfaction and postoperative recovery, it is important to identify strategies to reduce and prevent its occurrence. This scoping review seeks to explore the available evidence to synthesize the findings regarding the incidence of rebound pain and prevention strategies.
-
Methods
-
Research question
This scoping review sought to answer a two-part research question. First, we aimed to determine the incidence of rebound pain. Second, we explored effective prevention strategies.
Search Strategy
This scoping review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-ScR) checklist. Two research assistants with training in epidemiology and an anesthesiologist independently conducted a literature search from 2012 to December 2023, the databases consulted included Excerpta Medica dataBASE (EMBASE) and the National Library of Medicine (NHL) (PubMed). The databases were screened using the following terms: (postoperative pain OR rebound pain) AND (drug therapy OR analgesia) AND (regional anesthesia OR Nerve block). Both American and British spelling were considered.
-
Data selection and charting processes
The three chief investigators independently screened the databases for relevant articles. The first selection was based on the title and abstract. Duplicates were then removed. Subsequently, each investigator screened relevant records and performed a second selection. Finally, during a meeting, the remaining articles underwent a final screening and were included in the scoping review. Information Was Collected regarding the publication details(e.g., author and publication date) and study details(e.g., study design and assessments performed). The references were managed in Mendeley. We included cohort studies, transversal studies and clinical trials. We excluded studies concerning animal experiments, opinions, dissertations, letters to the editors and review articles.
-
Results
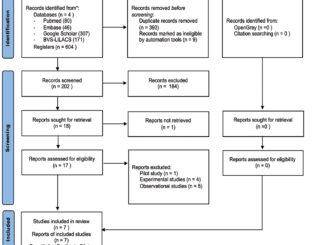
In total, 208 articles were identified in the initial review using the aforementioned search strategy. After eliminating 70 duplicate articles, the remaining 108 underwent further scrutiny based on the title and abstract, incorporating feedback from three peer reviewers. Initially, 30 articles were preselected; however, three were unavailable in full text, one was presented in a language other than English or Spanish, and one was identified as a research protocol as shown in Figure 1. Subsequently, 25 studies were subjected to thorough examination, leading to the exclusion of five publications that lacked data on the incidence of rebound pain or any strategies for its mitigation. In conclusion, 20 articles were ultimately included in this review, comprising 14 randomized clinical trials, four retrospective cohort studies, and one cross-sectional study (Table 1).
-
Definition of rebound pain
Rebound pain was initially characterized by Williams et al. in 2007 in a study involving patients who underwent anterior cruciate ligament reconstruction. It has been described as a form of surgical pain resulting from unopposed nociceptive inputs, which become apparent after the resolution of peripheral nerve block, or as a state of hyperalgesia emerging between 8 and 24 hours following the administration of the peripheral nerve block[5]. Over time, additional definitions of rebound pain have emerged, including its identification as acute, sudden, and significant postoperative pain that manifests after the resolution of a regional anesthesia intervention and significantly affects the quality of postoperative recovery[6]. Alternatively, it is defined as an acute yet often under-recognized escalation in pain severity following the dissipation of the effects of a peripheral nerve block (PNB)[7]-[8].
-
What is the incidence of rebound pain?
In 2018, it was reported that the incidence of rebound pain could reach 40% of patients in the resolution of PNB[9], however, when performing this review of the literature, an incidence of rebound pain was found from 6.67%[10] to 81%[8]. The results are summarized in Table 1.
4 articles are identified with the highest incidences of rebound pain:
In 2019, Thillainadesan T. et al., described a retrospective cohort of patients in whom an interscalene block was used as an analgesic method for shoulder surgery; 81% did report rebound pain[8].
Tayfun et al. in 2023, reported that when comparing patients undergoing shoulder surgery with a single-injection inter-scalene block versus patients with administration of intravenous dexamethasone, 30% reported rebound pain versus 73.3% of the control group that received only a single-injection interscalene block[6].
Goldstein et al, in 2022, demonstrated greater pain at 2, 4 and 8 hours postoperatively when patients received either general anesthesia (GETA) or intravenous sedation compared to those who received popliteal block, since this type of anesthesia provides effective pain control both in the intraoperative and postoperative. However, they report that the phenomenon of rebound pain following the use of regional anesthesia technique can be minimized with the timely use of oral narcotics and propose that patients begin taking oral pain medications 1 to 2 hours before regional anesthesia stops working[11].
In 2022, Admassie et al., report that the overall magnitude of rebound pain after the effect of peripheral nerve block was 61.7%[7].
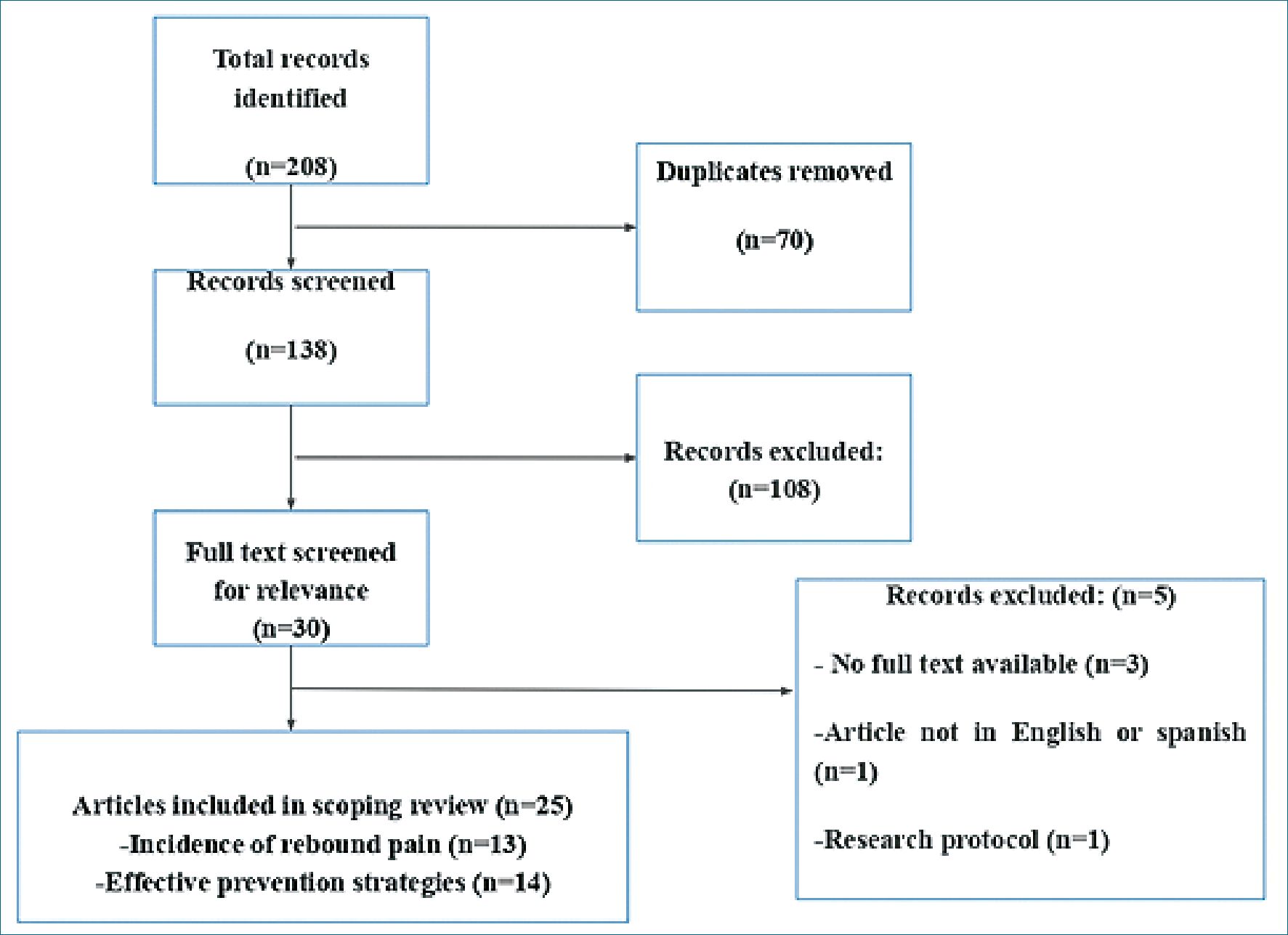
Figure 1. Preferred Reporting Items for Systematic reviews and Meta-Analyses(PRISMA) flow diagram for this scoping review on rebound pain.
-
Strategies used to avoid rebound pain
The described interventions to reduce rebound pain were: perineural dexamethasone, intravenous dexamethasone, perineural ketamine, intravenous ketamine, continuous perineural infusion, liposomal bupivacaine at the surgical site and transauricular vagus nerve stimulation.
Perineural dexamethasone
Fang J et al., published a randomized, double-blind clinical trial in 2021, in which they included 132 patients who underwent open reduction of an upper limb fracture, and were randomly assigned to peripheral nerve block with ropivacaine and dexamethasone 8 mg, compared to ropivacaine alone. They report that adding dexamethasone reduces rebound pain from 48.8% to 11%, also delays the onset of pain perception, and has a lower pain rate[13].
Other studies have reported similar data. Morita S. et al., In 2020, conducted a single-blind multicenter randomized clinical trial, which included 54 patients who underwent arthroscopic rotator cuff repair surgery and were randomly assigned to receive either an interscalene brachial plexus block with levobupi- vacaine and dexamethasone 3.3 mg, or levobupivacaine alone. They concluded that adding dexamethasone prolongs the duration of the block and decreases rebound pain[14].
Intravenous dexamethasone
In 2020, Holmberg A et al, published a double-blind randomized clinical trial, which included 51 participants who underwent distal radius fracture reduction, with regional anesthesia using a brachial plexus block, and were subsequently randomly assigned to receive 16 mg of intravenous dexamethasone compared to saline solution. The authors conclude that adding intravenous dexamethasone represented less rebound pain[24].
Later in 2023, Korkusuz et al., report a triple-blind randomized clinical trial, which included 60 patients undergoing for inguinal herniorrhaphy, in whom an iliohypogastric and ilioinguinal nerve block was performed. They were randomly assigned to receive 5 mg IV of dexamethasone compared to saline solution, showing that the dexamethasone group resulted in less rebound pain (6.67% vs. 50%, p < 0.001), as well as less opioid consumption[10].
Table 1. Overview of article relevant to the incidence of rebound pain
In the same year, Mingyang Gao et al reported a doubleblind randomized clinical trial, which included 130 patients undergoing open reduction of ankle fracture, in whom an adductor canal block was performed together with a popliteal sciatic block. Randomly assigned to receive the administration of 10 mg IV dexamethasone compared to saline solution, showing that the dexamethasone group reduces rebound pain (15% vs. 40%, p < 0.001), prolongs the effect of the block and presents improvement in quality of life[16].
Another randomized clinical trial published in 2023 by Tayfun et al., included 60 patients undergoing shoulder surgery, in whom an interscalene block was performed and the administration of 5mg IV dexamethasone was randomized. They report a lower incidence of rebound pain in the group that received dexamethasone (30% vs. 73.3%, p = 0.001)[6].
Similarly, Hyun Jung Lee et al., reported in 2023 a randomized clinical trial, which included 71 participants undergoing arthroscopic repair for a rotator cuff tear. The administration of dexamethasone 5 mg IV or perineural was randomly assigned, finding that the intravenous administration of dexamethasone has less rebound pain when compared to the perineural route (44.4%. vs. 20.0%, P = 0.028)[21].
Finally, in 2023, Touil N et al reported a retrospective cohort that compared the impact on rebound pain of intravenous dexamethasone according to dose. 118 participants received dexamethasone in low and high doses (< 0.1 mg/kg and > 0.1 mg/kg) after axillary plexus block, compared with a control group that didn’t receive dexamethasone. A decrease in rebound pain was found after an axillary plexus block with IV dexamethasone use (23% vs. 47%, p = 0.002), with a similar reduction regardless of the dose[23].
Perineural or intravenous ketamine
Tianqi Zhu et al., in 2020, reported a three-arm randomized clinical trial, which included 76 patients undergoing reconstruction of the anterior cruciate ligament, in whom a femoral and sciatic nerve block was performed. The first group received only ropivacaine, the second group was administered ropivacaine with perineural ketamine, and in the third group ropivacaine with intravenous ketamine was used. It was determined that adding perineural ketamine is related to less rebound pain (p = 0.001) and that intravenous ketamine does not represent any benefit[17].
Continuous local anesthetic infusion
Ding DY et al., in 2015, conducted a randomized clinical trial in which they included 60 patients with ankle fractures in whom a sciatic-popliteal block was performed with continuous infusion compared to a single injection. They concluded that continuous infusion reduces rebound pain and opioid consumption[20].
Contrary to these results, Rogero R. et al., reported in 2019 a randomized clinical trial, including 69 participants with foot and ankle fractures, in whom a popliteal block was performed with continuous infusion compared to a single injection. They identified that continuous infusion for popliteal nerve block didn’t reduce rebound pain evaluated at 72 hours using subjective analog pain scales (39.6 vs. 25.7, p = .044)[25].
Recently in 2023, Jong-Hyuk Lee et al., published a randomized clinical trial that included 66 participants in whom an infraclavicular brachial plexus block was performed after fixation of a distal radius fracture. Three intervention groups were randomized: single block with ropivacaine, single block with continuous perineural infusion, and single block with fentanyl patient-controlled analgesia (PCA). They concluded that adding continuous perineural infusion decreased rebound pain[18].
Local anesthetic at surgical site
In 2018, Namdari S. et al., reported a randomized clinical trial that included 78 patients undergoing shoulder arthroplasty, in whom an interscalene block was performed. They subsequently randomly assigned local application of liposomal bupivacaine to a group of patients at the surgical site. They conclude that the group that was administered local bupivacaine had more use of opioids and didn’t demonstrate a reduction in postoperative pain, which is why they describe a “double rebound” effect in these patients[22].
Transauricular vagus nerve stimulation
Zhou Qi et al., in 2022, published a double-blind randomized clinical trial, which included 78 participants in whom anterior cruciate ligament reconstruction was performed, randomly assigning a femoral block with transauricular stimulation of the vagus nerve, compared to a femoral block only. They describe that vagus nerve stimulation reduces the incidence of rebound pain (17.9% vs. 41%, p = 0.025), as well as reduces the need for additional analgesics in the postoperative period and the number of complications[15].
-
Factors associated with rebound pain
Factors independently associated with rebound pain after peripheral nerve block are younger age, female sex, surgery involving bone, and lack of perioperative use of intravenous dexamethasone[8],[9]. Additionally, in preoperative pain, the use of adjuvants such as lidocaine, the use of postoperative opioids, and NSAIDs decreases the likelihood of experiencing rebound after the peripheral nerve block has resolved[7].
However, regarding early age, Sort R. et al., found in 2018 that rebound pain was less in patients over 60 years. However, the majority reported moderate pain levels related to greater consumption of opioids during the period of rebound pain[26].
-
Discussion
In response to our initial question, when conducting this review, we found that there is still no consensus in the literature regarding the definition of rebound pain; however, due to this lack of homogeneity, there is a very wide variation in the incidence found by different authors between 6.67% and 80%. It is accepted in most studies that it is a pain that occurs between 8 and 24 hours after performing the peripheral nerve block, of moderate to high intensity, and that does not seem to have a subsequent relationship with the development of chronic pain. Most studies were performed in shoulder surgery (with interscalene blocks), foot and ankle surgery (with sciatic popliteal block), and others with various locations, such as femoral blocks, adductor canal, ilioinguinal, or iliohypogastric nerve blocks. Despite the lack of a robust study that compared the incidence of rebound pain between the different locations of surgery (shoulder, elbow, knee, ankle, etc.), multiple studies have suggested some risk factors associated with the more frequent presentation of this pathology, as evidenced in the results. These include being female, young, having intense preoperative pain, surgery involving bone, and the absence of intraoperative dexamethasone use.
Multiple strategies were compared for the prevention of rebound pain, among which the one that consistently showed the greatest decrease among the studies was the administration of intravenous dexamethasone. Different doses were compared without any significant differences in this aspect. Since all doses studied were effective in reducing rebound pain, it would be advisable to routinely administer intravenous dexamethasone in clinical practice with all peripheral nerve blocks. As long as there are no contraindications for its administration, more studies are required to define the ideal dose. Other techniques studied showed a possible benefit but with less consistency and with few studies still recommending their routine use, such as the use of perineural ketamine, NSAIDS, intravenous PCA pumps with opioids, transauricular stimulation of the vagus nerve, and the use of continuous infusion perineural catheters. However, further studies are required to confirm this benefit.
In conclusion, rebound pain is a pathology with a very high incidence in the population undergoing orthopedic surgery that must be explained to patients preoperatively, and an effort must be made to identify it postoperatively. There are multiple tools to try to reduce the incidence of rebound pain, and according to the evidence found in the studies carried out, intravenous dexamethasone should be used because it seems to significantly reduce the appearance of this pathology. The limitations of this scoping review include the limited number of studies that are available, whose main objective is rebound pain, and the lack of a homogeneous definition of the pathology and homogeneous cut-off points in terms of pain score, recovery time of the nerve block, appearance time of the rebound pain, as well as the difference in pre- and postoperative analgesic management that can generate variations in the duration of nerve blocks and appearance of said pathology. It must also be taken into account that most orthopedic surgeries are currently performed on an outpatient basis, and the resolution of nerve blocks occurs when the patient is already at home, which could generate an underreporting of the incidence of rebound pain and make it difficult to carry out studies due to inadequate follow-up of patients outside the hospital.
Declaration of generative al and al-assisted technologies in the writing process:
During the preparation of this work the author(s) used paperpal to correct possible grammatical errors, improve readability and language. After using this tool, we reviewed and edited the content as needed and take full responsibility for the content of this publication.
-
References
1. Dada O, Gonzalez Zacarias A, Ongaigui C, Echeverria-Villalobos M, Kushelev M, Bergese SD, et al. Does Rebound Pain after Peripheral Nerve Block for Orthopedic Surgery Impact Postoperative Analgesia and Opioid Consumption? A Narrative Review. Int J Environ Res Public Health. 2019 Sep;16(18):3257. https://doi.org/10.3390/ijerph16183257 PMID:31491863
2. Stone A, Lirk P, Vlassakov K. Rebound Pain After Peripheral Nerve Blockade-Bad Timing or Rude Awakening? Anesthesiol Clin. 2022 Sep;40(3):445–54. https://doi.org/10.1016/j.anclin.2022.03.002 PMID:36049873
3. Williams BA, Bottegal MT, Kentor ML, Irrgang JJ, Williams JP. Rebound pain scores as a function of femoral nerve block duration after anterior cruciate ligament reconstruction: retrospective analysis of a prospective, randomized clinical trial. Reg Anesth Pain Med. 2007;32(3):186–92. https://doi.org/10.1097/00115550-200705000-00003 PMID:17543812
4. Barry GS, Bailey JG, Sardinha J, Brousseau P, Uppal V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth. 2021 Apr;126(4):862–71. https://doi.org/10.1016/j.bja.2020.10.035 PMID:33390261
5. Marín B, Córdova S, Donoso M. Dolor de rebote en anestesia regional: una revisión narrativa. Rev Chil Anest. 2022;51(2):153–7. https://doi.org/10.25237/revchilanestv5104021159.
6. Et T, Basaran B, Bilge A, Yarımoğlu R, Korkusuz M, Tülüce İ. Rebound pain after interscalene brachial plexus block for shoulder surgery: a randomized clinical trial of the effect of different multimodal analgesia regimens. Ann Saudi Med. 2023;43(6):339–47. https://doi.org/10.5144/0256-4947.2023.339 PMID:38071444
7. Admassie BM, Tegegne BA, Alemu WM, Getahun AB. Magnitude and severity of rebound pain after resolution of peripheral nerve block and associated factors among patients undergoes surgery at university of gondar comprehensive specialized hospital northwest, Ethiopia, 2022. Longitudinal cross-sectional study. Ann Med Surg 2012 2022; 84: 104915
8. Thillainadesan T, Lee C, Mandaleson A, Hardidge A, Weinberg L, Tan C. Rebound pain after shoulder surgery with interscalene brachial Plexus blockade: How often? How bad? | Journal of Pain Management | EBSCOhost [Internet]. 2019 [cited 2023 Dec 29]. p. 147 Available from: https://openurl.ebsco.com/contentitem/gcd:137998141?sid=ebsco:plink:crawler&id=ebsco:gcd:137998141
9. Lavand’homme P. Rebound pain after regional anesthesia in the ambulatory patient. Curr Opin Anaesthesiol. 2018 Dec;31(6):679–84. https://doi.org/10.1097/ACO.0000000000000651 PMID:30124544
10. Korkusuz M, Basaran B, Et T, Bilge A, Yarimoglu R, Kurucay Y. The effects of dexamethasone added to ilioinguinal/iliohypogastric nerve (IIN/IHN) block on rebound pain in inguinal hernia surgery: a randomized controlled trial. Hernia. 2023 Dec;27(6):1571–80. https://doi.org/10.1007/s10029-023-02841-9 PMID:37477788
11. Goldstein RY, Montero N, Jain SK, Egol KA, Tejwani NC. Efficacy of popliteal block in postoperative pain control after ankle fracture fixation: a prospective randomized study. J Orthop Trauma. 2012 Oct;26(10):557–61. https://doi.org/10.1097/BOT.0b013e3182638b25 PMID:22732860
12. Jen TT, Ke JX, Wing KJ, Denomme J, McIsaac DI, Huang SC, et al. Development and internal validation of a multivariable risk prediction model for severe rebound pain after foot and ankle surgery involving single-shot popliteal sciatic nerve block. Br J Anaesth. 2022 Jul;129(1):127–35. https://doi.org/10.1016/j.bja.2022.03.030 PMID:35568510
13. Fang J, Shi Y, Du F, Xue Z, Cang J, Miao C, et al. The effect of perineural dexamethasone on rebound pain after ropivacaine single-injection nerve block: a randomized controlled trial. BMC Anesthesiol. 2021 Feb;21(1):47. https://doi.org/10.1186/s12871-021-01267-z PMID:33579199
14. Morita S, Oizumi N, Suenaga N, Yoshioka C, Yamane S, Tanaka Y. Dexamethasone added to levobupivacaine prolongs the duration of interscalene brachial plexus block and decreases rebound pain after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2020 Sep;29(9):1751–7. https://doi.org/10.1016/j.jse.2020.04.019 PMID:32815804
15. Zhou Q, Yu L, Yin C, Zhang Q, Tai Y, Zhu L Jnr, et al. Effect of Transauricular Vagus Nerve Stimulation on Rebound Pain After Ropivacaine Single Injection Femoral Nerve Block for Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial. J Pain Res. 2022 Jul;15:1949–58. https://doi.org/10.2147/JPR.S370589 PMID:35860416
16. Gao M, Li Y, Yu J, Li W, Qin S, Zhang Y, et al. The Effects of Intravenous Dexamethasone on Rebound Pain After Nerve Block in Patients with Ankle Fracture: A Randomized Controlled Trial. J Pain Res. 2023 Mar;16:1127–36. https://doi.org/10.2147/JPR.S399660 PMID:37025954
17. T Z. Y G, X X, S F, W L, J S. Effect of Ketamine Added to Ropivacaine in Nerve Block for Postoperative Pain Management in Patients Undergoing Anterior Cruciate Ligament Reconstruction: A Randomized Trial. Clin Ther [Internet]. Clin Ther. 2020;42:•••. [cited 2023 Dec 29] Available from: https://pubmed.ncbi.nlm.nih.gov/32247522/
18. Lee JH, Kim HJ, Kim JK, Cheon S, Shin YH. Does intravenous patient-controlled analgesia or continuous block prevent rebound pain following infraclavicular brachial plexus block after distal radius fracture fixation? A prospective randomized controlled trial. Korean J Anesthesiol. 2023 Dec;76(6):559–66. https://doi.org/10.4097/kja.23076 PMID:37089120
19. Ko SH, Park SH, Jang SM, Lee KJ, Kim KH, Jeon YD. Multimodal nerve injection provides noninferior analgesic efficacy compared with interscalene nerve block after arthroscopic rotator cuff repair. J Orthop Surg (Hong Kong). 2021;29(2):23094990211027974. https://doi.org/10.1177/23094990211027974 PMID:34278884
20. Ding DY, Manoli A 3rd, Galos DK, Jain S, Tejwani NC. Continuous Popliteal Sciatic Nerve Block Versus Single Injection Nerve Block for Ankle Fracture Surgery: A Prospective Randomized Comparative Trial. J Orthop Trauma. 2015 Sep;29(9):393–8. https://doi.org/10.1097/BOT.0000000000000374 PMID:26165259
21. Lee HJ, Woo JH, Chae JS, Kim YJ, Shin SJ. Intravenous Versus Perineural Dexamethasone for Reducing Rebound Pain After Interscalene Brachial Plexus Block: A Randomized Controlled Trial. J Korean Med Sci. 2023 Jun;38(24):e183. https://doi.org/10.3346/jkms.2023.38.e183 PMID:37337808
22. Namdari S, Nicholson T, Abboud J, Lazarus M, Steinberg D, Williams G. Interscalene Block with and without Intraoperative Local Infiltration with Liposomal Bupivacaine in Shoulder Arthroplasty: A Randomized Controlled Trial. J Bone Joint Surg Am. 2018 Aug;100(16):1373–8. https://doi.org/10.2106/JBJS.17.01416 PMID:30106818
23. Touil N, Pavlopoulou A, Delande S, Geradon P, Barbier O, Libouton X, et al. Effect of Intravenous Dexamethasone Dose on the Occurrence of Rebound Pain after Axillary Plexus Block in Ambulatory Surgery. J Clin Med. 2023 Jun;12(13):4310. https://doi.org/10.3390/jcm12134310 PMID:37445344
24. Holmberg A, Hassellund SS, Draegni T, Nordby A, Ottesen FS, Gulestøl A, et al. Analgesic effect of intravenous dexamethasone after volar plate surgery for distal radius fracture with brachial plexus block anaesthesia: a prospective, double-blind randomised clinical trial. Anaesthesia. 2020 Nov;75(11):1448–60. https://doi.org/10.1111/anae.15111 PMID:32472958
25. Rogero R, McDonald E, Raikin SM, Fuchs D, Shakked RJ, Voskeridjian A. Randomized Study of the Effect of Initial Ropivacaine Dosage during Continuous Popliteal Nerve Blocks on Rebound Pain in Foot and Ankle Surgery. Foot Ankle Orthop 2019; 4: 2473011419S00064 https://doi.org/10.1177/2473011419S00064.
26. Sort R, Brorson S, Gögenur I, Nielsen JK, Møller AM. Rebound pain following peripheral nerve block anaesthesia in acute ankle fracture surgery: an exploratory pilot study. Acta Anaesthesiol Scand. 2019 Mar;63(3):396–402. https://doi.org/10.1111/aas.13290 PMID:30411313

 ORCID
ORCID