Dr. Pablo Sepúlveda 1 , Dr. Alberto Laporte 1 , Dra. Andrea Fuentealba 1 , Dr. Luis Ignacio Cortínez 2
Rev Chil Anest Vol. 40 Núm. 2 pp. 122-137|doi:
PDF|ePub|RIS
Introducción
Our group has recently published a Propofol pharmacokinetic (PK) model including data obtained in normal weight and obese patients1. This model hasn’t been prospectively assessed in an obese population using target controlled infusion mode (TCI).
Objetivo General
The goal of this study was to evaluate the predictive performance of the model in a morbid obese population using TCI.
Material y Métodos
After Ethic Committee approval, patients proposed for bariatric surgery, standard monitoring, radial arterial line and BIS®, received a plasma TCI with the study propofol model to reach a target 12 µg/ml and then 0 until patients wake up (BIS > 75). Afterwards the patients received a TCI 4 µg/ml during 30 min followed by a target 2,5 µg/ml until the end of the surgery. Remifentanil and tracheal intubation with neuromuscular blocks were used after reaching propofol target of 4 µg/ml. Anestfusor® controlled the TCI pumps and stored the calculated plasma concentration (Cpcalc) throughout the study period. Arterial blood samples for propofol assays were collected at 1,2,3,5,9, 10,15,20,40,60,90 min, at stop of infusion, and at 1,3,5,10,30,60,120 min after stopping. The samples were analyzed in HPLC. The performance error for the population and for each anesthetic phase (induction, maintenance, recovery) were calculated using Varvel methodology2 (Median performance errors (MDPE)), Median absolute performance errors(MDAPE)), Wooble and divergence between the Cpcalc and the Cpmeasured.
Resultados
10 ASA I-II adult obese patients (50.2 ± 9.7 years, BMI 42 ± 6 kg/m2, Weight 118 ± 20 kg, 4 Female, 6 Male) were studied. Remifentanil was infused at a rate of 0,15 ± 0,06 µg/kg/min. We obtained 181 arterial blood samples of propofol. The global performance was poor, with an overprediction out of acceptable ranges (MDPE + 28%, MADPE 38%, Wooble 29%, divergence value of 22%). The induction phase showed a MDPE -7% and MADPE 19%, the recovery phase MDPE +4,6% and MADPE 11%. The maintenance phase showed the worst performance, with a MDPE +41% and MADPE of 41%.
Conclusiones
The integrated propofol PK model needs further adjustments before recommended it for TCI in morbidly obese patients.
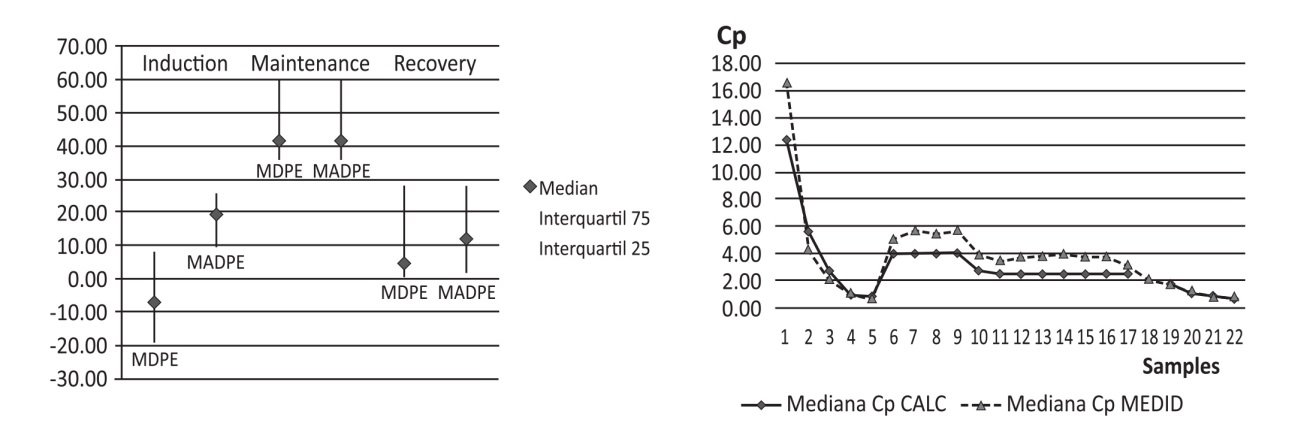
Figure 1 Performance error by phases (median-range) and by samples in median values.
