Dr. Mani Chandran 1 , Dr. Chris Hawthorne 1 , Professor Stefan Schraag 2 , Dr. Nick Sutcliffe 2
Rev Chil Anest Vol. 40 Núm. 2 pp. 122-137|doi:
PDF|ePub|RIS
Introducción
Using the Marsh model to deliver a target controlled infusion (TCI) of propofol, White derived a Covariates model which adjusts for age and sex as well as body weight1. Modifications were made to reflect reduced central compartment volume and clearance with increasing age, especially in females.
Objetivo General
We describe a prospective observational study with the primary objective of validating the Covariates model for propofol TCI. Secondary objectives include a comparison of venous and arterial blood sampling.
Material y Métodos
This is an ongoing study with a target recruitment of 30 patients. Prior consent is obtained from patients of ASA class I-II scheduled for elective surgery. Anaesthesia is induced using an Injectomat TIVA Agilia pump (Fresenius Kabi) programmed with the Covariates model for TCI propofol. Patients are randomized to a low-high-low (2-5-2 µ/ml) or high-low-high (5-2-5 µ/ml) schedule with plasma targets set and 0, 15 and 30 minutes. Venous and arterial blood samples are drawn at 1.5, 5, 16.5, 20, 31.5, 35 and 45-60 minutes post commencement of infusion. Propofol analysis is performed using high performance liquid chromatography. Performance error (PE) for each sample is calculated according to the equation: PE (%) = [measured]-[predicted]/[predicted] x 100. This allows calculation of median performance error (MDPE) as a measure of bias and median absolute performance error (MDAPE) as a measure of inaccuracy for each patient and overall.
Resultados
Pilot data from the first 4 patients is presented here. 1 female and 1 male patient were allocated to the low-high-low schedule and 2 males to the high-low-high schedule. Age range was 29-54 years and weight range 66-117 kg. Venous and arterial samples were collected at all predetermined time points except in patient 2 where the 45-60 minute sample was omitted. Figure 1 shows the PE for venous sampling plotted against time for each patient. For venous sampling, the overall MDPE was 4.3% (-11,5-29,2) and MDAPE 24.0% (1,0-54,2). For arterial sampling, the overall MDPE was 33,1% (7,3-59,0) and MDAPE 39,8% (9,7-59,0).
Conclusiones
This study is designed to validate the Covariates model for propofol TCI as it will be used in clinical practice with multiple step changes in plasma targets. The preliminary data presented suggest an acceptable level of bias (4,3%) and inaccuracy (24,0%) when assessed on venous samples. The trend with arterial sampling is towards increased bias and inaccuracy.
Referencias
-
White M, Kenny GN, Schraag S: Use of target controlled infusion to derive age and gender covariates for propofol clearance. Clin Pharmacokinet. 2008; 47: 119-2.
This study is funded by the Department of Anaesthesia and Peri-operative Medicine at the Golden Jubilee National Hospital.
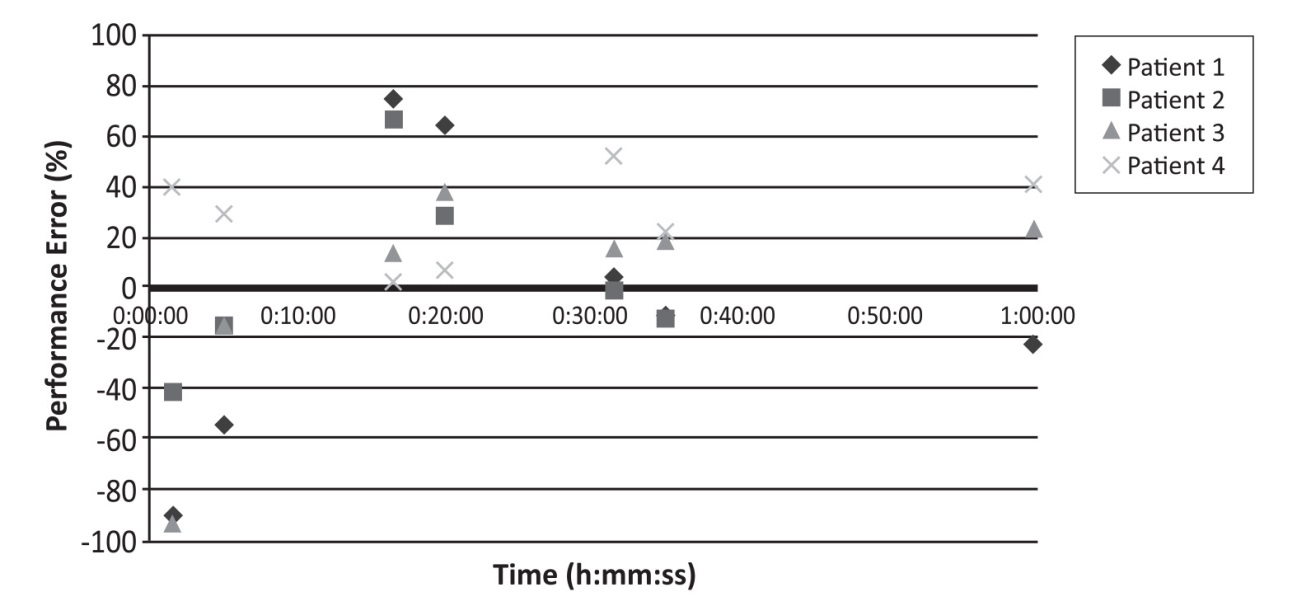
Figure 1 Performance error for venous samples.


 Creative Commons Attribution
Creative Commons Attribution