Matías Ramos 1 ,*, Franco Fratebianchi 1 , Stella Verlangieri 1
Recibido: 24-03-2021
Aceptado: 03-05-2021
©2021 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 50 Núm. 6 pp. 886-889|https://doi.org/10.25237/revchilanestv50-03-03
PDF|ePub|RIS
Shock vasopléjico luego de la resección de un fecromocitoma: el rol del azul de metileno
Abstract
We present a case of a patient with hypotension refractory to the administration of norepinephrine after laparoscopic pheochromocytoma resection in the left adrenal gland. We describe the use of methylene blue as an alternative drug therapy to the adrenergic system, as well as associated doses, contraindications and complications.
Resumen
Presentamos el caso de un paciente con hipotensión refractaria a la administración de noradrenalina luego de la resección laparoscópica de un feocromocitoma en la glándula suprarrenal izquierda. Describimos el uso de azul de metileno como terapia farmacológica alternativa al sistema adrenérgico, así como también dosis, contraindicaciones y complicaciones asociadas.
-
Introduction
-
Case presentation
The perioperative management of pheochromocytoma is a challenge for the perioperative medical team. From preoperative optimization to management of adrenergic crises in the operating room, the role of the anesthesiologist is crucial in order to obtain good results. In particular, hypotension after surgical isolation of the tumor can be a problem given its refractoriness to commonly used vasoactive agents. We will describe the use of methylene blue in a case of vasoplegic shock refractory to norepinephrine.
A 56-year-old, male ASA-III patient presented with an adrenal tumor identified as pheochromocytoma, scheduled for laparoscopic adrenalectomy. At the pre-anesthetic visit, he revealed a history of heart failure, atrial fibrillation, arterial hypertension, diabetes mellitus, hypothyroidism, myocardial infarction that required placement of a metal stent in the right coronary artery, and type I neurofibromatosis. His usual medications included: valsartan, ivabradine, metformin, digoxin, rosuvastatin, clopidogrel, nebivolol, levothyroxine, acetylsalicylic acid, doxazosin, and acenocoumarol. Acenocoumarol and clopidogrel were suspended five days before surgery, according to current recommendations[1]. Preoperative studies included an echocardiogram that reveals global hypokinesia, eccentric ventricular hypertrophy, ejection fraction of 38%, decreased right ventricular function (14 mm TAPSE), and mild mitral regurgitation. Coronary angiography shows the anterior descending artery free of lesions, the circumflex artery with a severe lesion that compromises its lumen by 70% and the right coronary artery with severe stent restenosis that decreases its lumen by 95%. On the day of surgery, the blood pressure on admission to the operating room was 132/76 mmHg and the heart rate was 86 beats per minute (bpm). He was premedicated intravenously with midazolam (2 mg) and fentanyl (75 pg), basic monitors were placed and the radial artery was catheterized. After induction with fentanyl (6 pg kg-1), propofol (1 mg kg-1) and rocuronium (0.6 mg kg-1), an ultrasound-guided central venous catheter was placed in the right internal jugular. Remifentanil (0.4 pg kg-1 min-1) and sevoflurane (% et sevoflurane 1.4) were used for maintenance of anesthesia.
During surgery, mean arterial pressure (MAP) was maintained between 70 and 90 mmHg with an infusion of norepinephrine (initial dose 0.033 pg kg-1 min-1). Due to surgical manipulation, the tumor released catecholamines causing hypertensive crises that required the administration of sodium nitroprusside in infusion titrated between 1 to 2.5 pg kg-1 min-1 plus boluses of phentolamine of 2 mg. There was an episode of sinus tachycardia of 145 bpm associated with 270/130 mmHg of blood pressure that required the administration of 0.5 mg kg-1 of esmolol.
Minutes after clamping of the effluent vein, a hypotensive crisis precipitated. All vasodilators were suspended and the norepinephrine infusion was restarted at 0.15 pg kg-1 min-1, which, given the lack of response, was titrated to a dose of 0.7 pg kg-1 min-1. MAP continued at 40 mmHg despite fluid therapy with lactated Ringer and there was no response to boluses of 50 pg of norepinephrine.
Although the adrenergic discharges produced by the release of catecholamines by the tumor configure a cardiac challenge, which in the context of an organ damaged by ischemic heart disease could be a trigger for cardiogenic collapse, hypotension, tachycardia and low central venous pressure (4 mmHg) was interpreted as vasoplegic shock. In favor of this interpretation is the surgical context and the temporal correlation with the vascular isolation of the tumor, the absence of electrocardiographic signs of cardiac ischemia, and the absence of pulmonary changes suggestive of acute lung edema.
Given the lack of vasopressin in our institution and given the evident inefficiency of adrenergic agonists for the control of blood pressure, it was decided to administer methylene blue, 10 mg bolus and then 60 mg more as an infusion over 10 minutes. Once the administration was finished, blood pressure began to gradually recover during surgical closure and it was necessary to decrease the dose of norepinephrine. After the surgery, the patient was extubated in the operating room and the vasoactive drugs were suspended. He was transferred to the Coronary Unit lucid and hemodynamically stable. His vital signs upon admission were: HR 107 bpm, BP 120/70 (89) mmHg, SpO2 95% in ambient air and 18 breaths per minute. The patient was dis- charged from the closed unit three days later.
-
Discusion
From a reductionist perspective, the ultimate goal of the cardiovascular and respiratory systems can be understood as a unique apparatus whose task is to provide a relatively constant supply of oxygen to the tissues. This variable, known as the oxy- gen delivery (DO2), depends on the cardiac output (carried out by the cardiovascular system) and on the concentration of oxy- gen in the blood (carried out by the respiratory system). With this model, circulatory shock can be defined as acute circulatory failure, where the body is unable to provide an adequate oxygen delivery due to a failure in the cardiovascular system[2]. Classically, circulatory shock is classified according to the affected determinant of cardiac output: if the failure is in preload, it is called hypovolemic shock; if it is in afterload, obstructive shock; and if it is in contractility, cardiogenic shock. To this classification is added one more category: the loss of vascular tone. In certain situations and for different reasons, there is great vasodilation and the consequent loss of peripheral resistance. The end result is a drop in afterload, which, even with normal or even increased cardiac output, the body loses the ability to generate adequate blood pressure for perfusion.
The word vasoplegia describes, from its etymological root, the state of unresponsiveness to vasopressors. Its hemodynamic profile includes hypotension, high cardiac output, low peripheral resistance, and low filling pressure despite the infusion of high doses of vasopressors[3].
Once the diagnosis of vasoplegic shock is made, it is important to act quickly, since it is a condition associated with poor prognosis and high postoperative morbidity and mortality[4] Aggressive therapy is required and the anesthesiologist has pharmacological tools to deal with it. These tools were classified according to a recent and comprehensive review by Chow et al[5] into first, second and third line drugs, followed by rescue drugs and emerging drugs.
Norepinephrine is currently considered the first-line drug for the treatment of vasoplegic shock. The powerful vasoconstrictor action of this catecholamine is mediated by a1 receptors in vascular smooth muscle. It is known that as the norepinephrine dose increases, desensitization and tachyphylaxis appear due to the phosphorylation and internalization of a1 receptors. Once this state is reached, successive increases in the vasopressor dose will not be able to increase blood pressure and here the need to start second-line drugs arises.
According to current evidence, vasopressin is considered a second-line drug, an agent that acts through vascular V1 receptors (causing vasoconstriction) and V2 receptors in the kidneys (generating free water retention). In severe occasions where norepinephrine and vasopressin fail to stabilize hemodynamics, a third-line drug may be considered. In this category is adrenaline. Since adrenaline uses the same vasoconstrictive mechanism as norepinephrine, its use is limited. Unless myocardial dysfunction is considered a concomitant cause of shock and inotropic support is required, the use of epinephrine would not be optimal. Anaphylaxis situations represent a special case, since it is adrenaline that reverses the pathophysiological mechanisms of anaphylactic shock. Finally, we have the rescue and emerging drugs. As rescue agents we have methylene blue, thiamine, and steroids.
In this case report we show the effect of an abrupt cut in the supply of endogenous catecholamines by the pheochromocytoma after its surgical isolation. The sudden decrease in the concentration of catecholamines, the decrease in adrenergic receptors on the cell surface, and the residual action of adrenergic antagonists caused a hypotensive crisis that required the rapid intervention of the medical team. Most of the time, the body responds to the suspension of vasodilators, adequate fluid therapy, and the infusion of exogenous catecholamines. In this case, aggressive use of adrenergic drug therapy with norepinephrine was completely ineffective, leading to the diagnosis of catecholamine-refractory vasoplegic shock[6].
The use of high-dose catecholamines is associated with adverse effects, including modulation of the inflammatory response, increased oxidative stress, and myocardial, mesenteric, and digital ischemia[7],[8]. Norepinephrine doses greater than 1 ug kg-1 min-1 are associated with high mortality. As a consequence, alternatives must be considered and thus the concept called “decatecholaminization” arises, where non-catecholaminergic vasopressors are used to reduce exposure to catecholamines[9]. Pharmacological alternatives were immediately considered, and vasopressin was a great candidate[10], due to its lack of availability in our institution, it was ruled out.
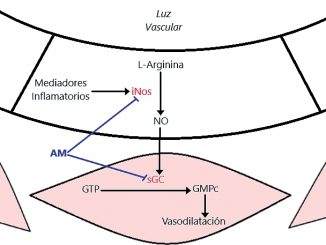
We know that surgical stress and inflammatory phenomena promote the activation of the inducible isoform of nitric oxide synthase (iNOS) in the vascular endothelium. This enzyme, of which there are three isoforms (nNOS, eNOS and iNOS), catalyzes the reaction that produces nitric oxide (NO) from L-arginine. The produced NO diffuses into the muscle layer, which is part of the wall of the microcirculation, and there activates the enzyme guanylate cyclase (GC)[11]. GC catalyzes the reaction by which cyclic guanosine triphosphate (GTP) forms cyclic guanosine monophosphate (cGMP). This latter mediator participates as a second messenger, activating kinases and finally promoting the decrease in intracellular calcium concentration. Calcium plays a fundamental role as a trigger for smooth muscle
contraction, so its decrease leads to the reverse phenomenon: smooth muscle relaxation. This, in the vascular tree, translates as vasodilation and the consequent loss of peripheral vascular resistance (Figure 1). With such a low afterload, the ability to generate blood pressure is compromised.
Methylene blue has a particular and unique mechanism of action, as it raises pressure not by causing vasoconstriction of vascular smooth muscle, but by preventing vasodilation. This drug is not a vasoconstrictor and does not depend on the availability of adrenergic receptors for the recovery of muscle tone since it crosses cell membranes and acts by inhibiting iNOS and GC in vascular smooth muscle[12]. This dye, outside of the perioperative context, has been used for the treatment of methemoglobinemia, priapism and malaria, showing an adequate safety profile for its use. In the field of critical care and anesthesia, methylene blue has proven to be useful in vasoplegia in cardiac surgery[13], in reperfusion of liver transplantation, in anaphylactic shock and in burn patients[14].
The dose of methylene blue usually used according to re- search on septic shock, cardiac surgery or anaphylaxis is 1 to 2 mg kg-1[15]. The hemodynamic effects of methylene blue are relatively rapid and it is eliminated as leucomethylene blue in feces, bile, and urine. Toxic manifestations have been reported with doses of 7 mg kg-1 and the lethal dose is 40 mg kg-1. After ruling out contraindications, such as the use of monoamine oxidase inhibitors or glucose 6 phosphatase deficiency, it was decided to administer 1 mg kg-1 of methylene blue. This produced a substantial improvement in the hemodynamic profile, which manifested in the need to decrease the dose of norepinephrine as blood pressure recovered to normal values. The result was similar to that obtained in a previous report, but in a pediatric case[16].
Methylene blue, like deoxyhemoglobin, absorbs the 660 nm wavelength light emitted by the saturometer. This can be interpreted by the device as arterial desaturation[17]. In our case, this phenomenon was not observed.
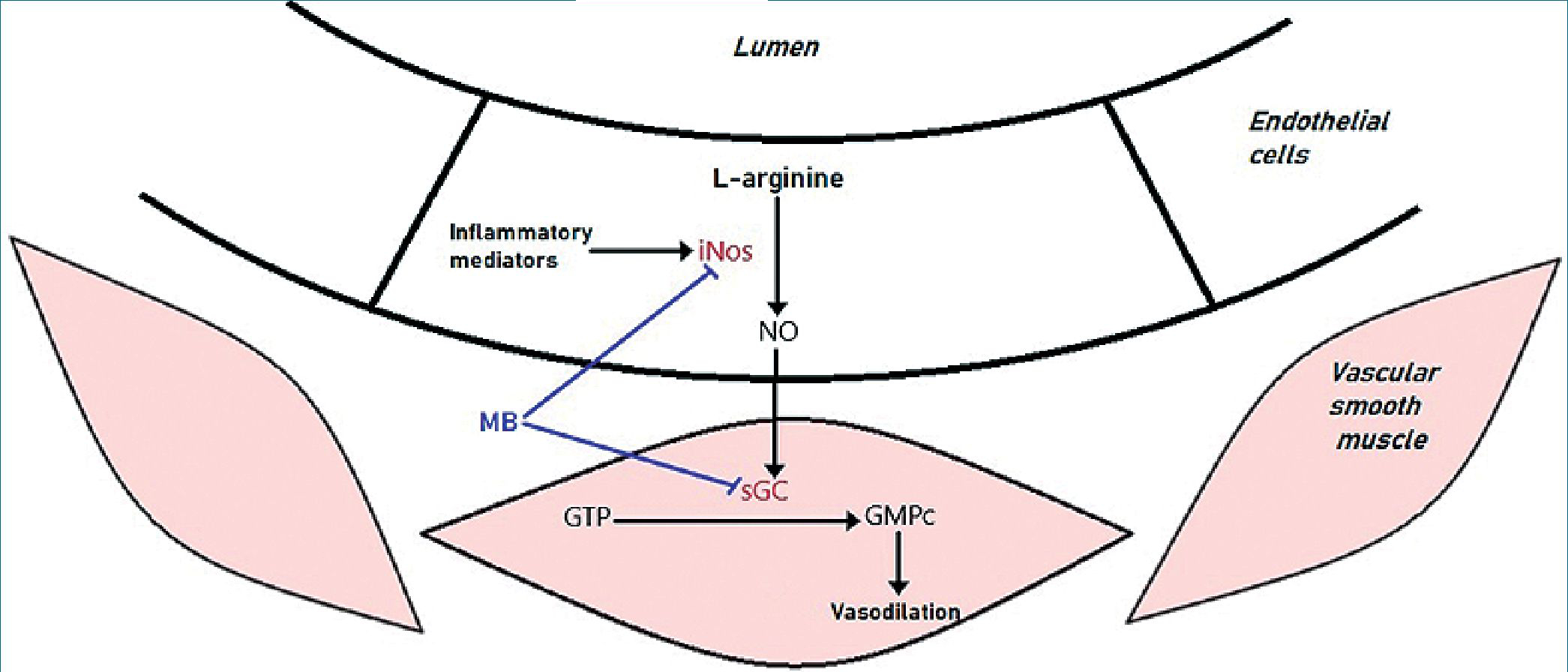
Figure 1. Mechanism of action of methylene blue. For references see text
-
Conclusion
This case report shows the hemodynamic response to methylene blue of a catecholamine-refractory vasoplegic shock secondary to pheochromocytoma resection. We believe that the improvement was not just a coincidental correlation, but higher quality research should be done to provide better evidence. We are sure that the anesthesiologist must have a low threshold to start therapy with pharmacological alternatives to the adrenergic system in order to achieve hemodynamic stability once hypovolemia is corrected. However, scientific evidence supporting the use of methylene blue is not of high quality, we believe that this agent should not be used as a first treatment option but it should be considered as a safe and rational option.
We hope that the report of this case, with others, will be the basis for the development of trials of greater complexity and scientific quality in the future in order to obtain better clinical evidence.
-
Declarations
The authors declare that the informed consent of the patient has been obtained for the publication of this work. The authors confirm that the data supporting the findings of this study are available within the article (and/or) its supplementary materials. Finally, the authors declare no competing interests and no specific funding for this work.
Soporte financiero: Nulo.
Conflicto de interés: No hay conflicto de intereses.
References
1. Kozek-Langenecker, S., Ahmed, A., Afshari, A., Albaladejo, P., Aldecoa, C., & Barauskas, G. et al. (2017). Management of severe perioperative bleeding. Eu-ropean Journal Of Anaesthesiology, 34(6), 332-395. https://doi.org/10.1097/EJA.0000000000000630
2. Landry DW, Oliver JA. The pathogenesis of vasodilatory shock.N Engl J Med 2001;345:588-95. https://doi.org/10.1056/NEJMra002709
3. Augoustides J, Abrams M, Berkowitz D. Vasopressin for hemodynamic rescue in catecholamine-resistant vasoplegic shock after resection of massive pheochromocytoma. Anesthesiology 2004; 101: 1022-1024. https://doi.org/10.1097/00000542-200410000-00031
4. Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol. 1993;45: 367-374. https://doi.org/10.1016/0006-2952(93)90072-5
5. Faber P, Ronald A, Millar BW. Methylthioninium chloride: pharmacology and clinical applications with special emphasis on nitric oxide mediated vasodilatory shock during cardiopulmonary bypass. Anaesthesia 2005; 60: 575-587 https://doi.org/10.1111/j.1365-2044.2005.04185.x
6. Leyh RG, Kofidis T, Strüber M, Fischer S, Knobloch K, Wachsmann B, Hagl C, Simon AR, Haverich A. Methylene blue: the drug of choice for catecholami-ne-refractory vasoplegia after cardiopulmonary bypass? J Thorac Cardiovasc Surg 2003;125:1426-31) https://doi.org/10.1016/S0022-5223(02)73284-4
7. Hosseinian, L., Weiner, M., Levin, M. A., & Fischer, G. W. (2016). Methylene Blue. Anesthesia & Analgesia, 122(1), 194-201. https://doi.org/10.1213/ANE.0000000000001045
8. McCartney, S. L., Duce, L., & Ghadimi, K. (2018). Intraoperative vasoplegia: methylene blue to the rescue! Current Opinion in Anaesthesiology, 31(1), 43-49. https://doi.org/10.1097/ACO.0000000000000548
9. Amin Nasr, A., Fatani, J., Kashkari, I., Al Shammary, M., & Amin, T. (2009). Use of methylene blue in pheochromocytoma resection: case report. Pediatric Anesthesia, 19(4), 396-401. https://doi.org/10.1111/j.1460-9592.2009.02956.x
10. Kessler MR. Spurious pulse oximeter desaturation with methylene blue injection. Anesthesiology 1996;65:435-6. https://doi.org/10.1097/00000542-198610000-00017

 ORCID
ORCID

 Creative Commons Attribution
Creative Commons Attribution