Yannis Amador Godoy MD. 1 ,a, María Servito BHSc. 2 ,a, Joel Parlow MD, MSc, FRCPC. 2
Recibido: 02-05-2021
Aceptado: 12-06-2021
©2021 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 50 Núm. 6 pp. 894-897|https://doi.org/10.25237/revchilanestv5007101119
PDF|ePub|RIS
Oclusión de arteria coronaria y falla ventricular derecha luego de reemplazo valvular aórtico
Abstract
A 50-year-old female presented for an elective aortic valve replacement (AVR). After a successful AVR and repair of a pulmonary vein injury during the vent placement, a difficult wean from cardiopulmonary bypass (CPB) was presented with an acute right ventricle (RV) failure. We review acute RV failure and consequent difficult wean from CPB. Written consent was obtained for this paper.
Resumen
Se presenta el caso clínico de una paciente femenina de 50 años sometida a recambio valvular aórtico presenta un destete complejo de circulación extracorpórea debido a falla ventricular derecha.
-
Glossary of terms
2D Two-dimensional
AS Aortic Stenosis
AVR Aortic Valve Replacement
CABG Coronary Artery Bypass Graft
CPB Cardiopulmonary Bypass
ECG Electrocardiogram
IABP Intra-aortic Balloon Pump
RCA Right Coronary Artery
RV Right Ventricular
TEE Transesophageal Echocardiogram
-
Introduction
Post-operative acute right ventricular (RV) failure is associated with 75% in-hospital mortality[1]. The right ventricle is a delicate structure susceptible to dysfunction imbued by trauma during cardiac surgery. Although optimal delivery of cardioplegia prevents post-operative acute RV failure2is more likely as-sociated with cardiovascular and pulmonary complications related to cardiopulmonary bypass (CPB, it can still occur. Early recognition and immediate management are paramount to its resolution[2]. Yet, clear guidelines regarding its diagnosis and treatment are lacking. For instance, effectiveness of intra-aortic balloon pump (IABP) in post-operative acute RV failure remains controversial[2]-[5] Herein, we describe how we recognized and successfully managed post-operative acute RV failure. Informed consent was obtained from the patient and this manuscript adheres to the EQUATOR guidelines.
-
Case report
A 50-year-old female with critical aortic stenosis (AS) due to calcification presented for an elective aortic valve replacement (AVR). She had no other significant past medical history. On the pre-operative coronary angiogram, there was evidence of 90% stenosis in the first diagonal branch, for which medical management was decided. Otherwise, the coronaries appeared normal with very minimal disease. Left ventricular ejection fraction was 71% on the two-dimensional transesophageal echo-cardiogram (TEE; Supplementary Video 1).
The patient underwent uneventful induction with a general anesthetic, endotracheal intubation, arterial line cannulation, and central line access. A standard thoracotomy was performed with aorta cannula placement and atrial cannulation. While placing the right superior pulmonary vent an inadvertent perforation occurred and was identified. The institution of cardio- pulmonary bypass (CPB) was started and antegrade cardioplegia was delivered. A bioprosthetic aortic valve (SOLO Stentless, 21mm) was subsequently placed. Before coming off CPB, the pulmonary vein perforation was closed. The weaning process was initiated and once the heart was filled, the implanted valve was assessed with TEE. The position, motion and gradient of the bioprosthetic valve were appropriate. However, we experienced a difficult wean from CPB, accompanied by hypotension and acute right ventricular (RV) failure. On TEE, the RV was enlarged and dyskinetic at the base, but normal at the apex (Figure 1A-D; Supplementary Video 2).
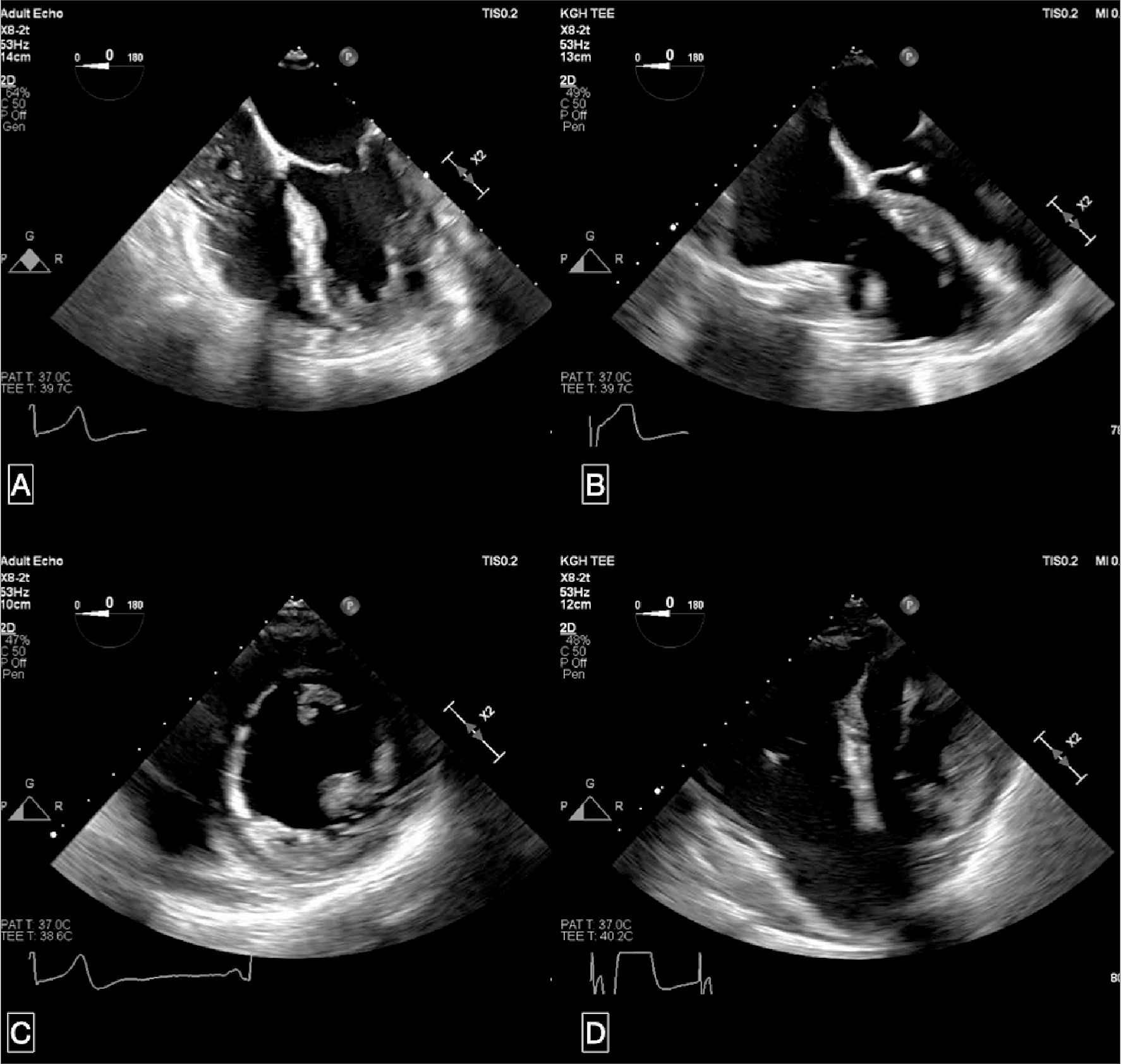
Figure 1. Representative images of the transoperative echocardiogram (TOE) of the right ventricle after induction and after coming off-pump. Panel A shows the trans-gastric mid-papillary short-axis view after induction, while Panel B shows the image coming off-pump. The difference in right ventricular (RV) size and diastolic flattening of the septum into the left ventricle can be noted in Panel A and B. Panel C shows the mid-esophageal four-chamber view after induction, while Panel D shows the image coming off-pump. Difference in RV sizes can be noted between Panel C and D, with the latter representing basal hypokinesia.
A decision was made to re-institute CPB to promote RV recovery, unloading the chamber and initiating inotropic support with epinephrine, norepinephrine, milrinone, vasopressin, and epoprostenol. Despite these measures, the patient remained hemodynamically unstable. An IABP was inserted to reinforce the RV and assist with the CPB wean. Eventually, the patient stabilized and was transferred to the cardiac recovery unit, with profound reliance on mechanical and inotropic support: epinephrine at 8 mcg/min, norepinephrine at 2mcg/min, milrinone at 0.375 mcg/kg/min, vasopressin at 0.06 mcg/min, and inhaled epoprostenol.
A 12-lead electrocardiogram (ECG) in the unit showed a J point and ST segment elevation in leads II, III and aVF, with reciprocal changes in V1 and aVR (Figure 2). A coronary angio- gram performed three hours after arrival in the recovery unit revealed a subtotal occlusion of the ostial right coronary artery (RCA, Figure 3; Supplementary Video 3). In fact, there was significant difficulty in the insertion of the guide wire. An aortography was performed to assess its patency, which revealed slow flow. Given the unstable hemodynamics, a likely injury to the ostial RCA and recent sternotomy, a decision was made for an urgent off-pump coronary artery bypass graft (CABG) procedure, using a saphenous vein graft to the right coronary artery. Following CABG, the TEE showed enhancement of the RV performance. The clinical improvement was also demonstrated with a reduction of the IABP ratio. The patient was transferred to the cardiac recovery unit on substantially reduced inotropic support; specifically, epinephrine and norepinephrine at 3 mcg/min and 1 mcg/min, respectively. The RV function continued to improve. The mechanical support by IABP was stopped within the first 12 hours of arrival, and inotropic support was discontinued on postoperative day 1. She was discharged home with stable vital signs on a postoperative day 6.
-
Discussion
Postoperative acute refractory RV failure is associated with up to 70-75% in-hospital mortality, as opposed to 0.1% of patients after routine cardiac surgery.[1] This is often caused by myocardial ischemia and depression after CPB in susceptible patients[1]. The RV is a delicate structure that is affected by different pathways, including pulmonary pressure, volume status, geometry, and ventricular interdependence. Surgical interventions produce a negative effect on the RV the moment the pericardium is incised.6
Prevention can be achieved through optimal myocardial protection using cardioplegia, with antegrade delivery superior to retrograde.[2],[4]. The use of cardioplegia has indeed contributed to RV infarction being extremely rare[2]. Despite optimal cardioplegia, RV infarction and dysfunction can still occur, potentiating and perpetuating post-operative acute RV failure[3]. The early recognition of RV failure is paramount to its management, for treatment delay results in poor prognosis due to the rapid hemodynamic decline and irreversible damage to the RV myocardium[2]. However, early recognition can be complicated by patients’ comorbidities[2]. Clinical signs such as hypotension are often non-specific[2], but ECG, albeit underestimated, can be useful in the diagnosis of acute RV failure. In our case, a 12-lead ECG on arrival in the recovery unit showed clear RV involvement as an elevation of the J point throughout inferior leads. Noteworthy, the ST elevation in lead III > lead II suggested an RCA occlusion7, which was precisely confirmed on angiogram.
Another crucial element to our successful management of acute RV failure was the use of TEE. There are several ways of measuring the right-sided heart, but TEE measurements prior to pericardiotomy have not been validated for prognostication. Al- though two-dimensional (2D) analyses have demonstrated a re- duction in longitudinal shortening and an increase in transverse shortening, there is no association between initial assessment of RV function and degree of RV failure[6],[8]. In contrast, the free wall RV strain can detect subclinical dysfunction and thus, is the only promising parameter for predicting RV failure[8]. Herein, free wall RV strain measured intraoperatively proved to be beneficial as it provided further evidence that the cause of hemodynamic instability was acute RV failure.
The literature is sparse regarding the appropriate management of postoperative acute RV failure[2]; randomized clinical trials and/or observational studies are lacking. The utility of IABP in postoperative acute RV failure is also controversial, with a paucity of data regarding its efficacy and escalation to a right ventricular assist device is often necessary[2]-[5].
In addition to early detection, herein we describe the successful treatment of postoperative acute RV failure. We attained stable hemodynamics with inotropic support and institution of IABP, allowing us to do further investigations to ascertain and reverse the cause. In our case, a coronary angiogram was per- formed due to suspected RV infarction. An occlusion of the ostial RCA was found, and the patient underwent a re-do sternotomy for revascularization of her RCA.
Not only did the patient have minimal coronary artery dis- ease on the pre-operative angiogram, she did not have risk factors that would render her susceptible to a difficult CPB wean[9],[10]. Therefore, we suspect that the occlusion was iatrogenic caused intraoperatively. Consequently, delivery of cardioplegia to the RV may have been impaired, leading to insufficient myocardial protection and thus, potentiation of acute RV failure. The patient underwent urgent revascularization. Due to the rapid reversal of cause, RV function improved post-operatively without significant impairment in RV function.
-
Conclusion
Good communication between the anesthesiologist, surgeon and perfusionist is paramount to the detection of abrupt and unexpected RV failure. RV failure after CABG, albeit rare, can occur due to poor RV protection or an underlying undiagnosed coronary deleterious blood flow. Accurate prediction on RV failure is not yet feasible, but a reduced RV strain might provide some clue for the subclinical disfunction RV cases.
Video 1: SAX https://vimeo.com/manage/videos/640523125
Video 2: ME 4C https://vimeo.com/manage/videos/640521547
Video 3: Angiogram https://vimeo.com/manage/videos/640522430
References
1. Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: Ii. pathophysiology, clinical importance, and management. Anesth Analg. 2009;108(2):422-433. https://doi.org/10.1213/ane.0b013e31818d8b92
2. Estrada VHN, Franco DLM, Moreno AAV, Gambasica JAR, Nunez CCC. Postoperative Right Ventricular Failure in Cardiac Surgery. Cardiol Res. 2016;7(6):185-195. https://doi.org/10.14740/cr500e
3. Wanner PM, Filipovic M. The Right Ventricle-You May Forget It, But It Will Not Forget You. J Clin Med. 2020;9(2):432. https://doi.org/10.3390/jcm9020432
4. Vlahakes GJ. Right Ventricular Failure After Cardiac Surgery. Cardiol Clin. 2012;30(2):283-289. https://doi.org/10.1016/j.ccl.2012.03.010
5. Krishnamoorthy A, DeVore AD, Sun JL, et al. The impact of a failing right heart in patients supported by intra-aortic balloon counterpulsation. Eur Hear journal Acute Cardiovasc care. 2017;6(8):709-718. https://doi.org/10.1177/2048872616652262
6. Rong LQ, Yum B, Abouzeid C, et al. Echocardiographic predictors of intraoperative right ventricular dysfunction: A 2D and speckle tracking echocardiography study. Cardiovasc Ultrasound. 2019;17(1):1-8. https://doi.org/10.1186/s12947-019-0161-3
7. Fiol M, Cygankiewicz I, Guindo J, et al. Evolving Myocardial Infarction with ST Elevation: Ups and Downs of ST in Different Leads Identifies the Culprit Artery and Location of the Occlusion. Ann Noninvasive Electrocardiol. 2004;9(2):180-186. https://doi.org/10.1111/j.1542-474X.2004.92538.x
8. Lemarié J, Huttin O, Girerd N, et al. Usefulness of Speckle-Tracking Imaging for Right Ventricular Assessment after Acute Myocardial Infarction: A Magnetic Resonance Imaging/Echocardiographic Comparison within the Relation between Aldosterone and Cardiac Remodeling after Myocardial Infarction. J Am Soc Echocardiogr. 2015;28(7):818-827.e4. https://doi.org/10.1016/j.echo.2015.02.019
9. Salis S, Mazzanti V V., Merli G, et al. Cardiopulmonary Bypass Duration Is an Independent Predictor of Morbidity and Mortality After Cardiac Surgery. J Cardiothorac Vasc Anesth. 2008;22(6):814-822. https://doi.org/10.1053/j.jvca.2008.08.004
10. Denault AY, Tardif JC, Mazer CD, Lambert J. Difficult and complex separation from cardiopulmonary bypass in high-risk cardiac surgical patients: A multicenter study. J Cardiothorac Vasc Anesth. 2012;26(4):608-616. https://doi.org/10.1053/j.jvca.2012.03.031

 ORCID
ORCID
 Creative Commons Attribution
Creative Commons Attribution