Sergio Gallego Zarzosa 1 ,*, Cruz Soriano Cuesta 1 , Susana García Plaza 1 , Juan Higuera Lucas 1 , Raúl de Pablo Sánchez 1
Recibido: 19-04-2021
Aceptado: 25-06-2021
©2022 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 51 Núm. 2 pp. 213-216|https://doi.org/10.25237/revchilanestv5109021126
PDF|ePub|RIS
Uso compasivo de cefiderocol en el tratamiento de pseudomona multirresistentes en paciente crítico
Abstract
Background: One of the newest antibiotics against multidrug-resistant (MDR) bacteria is Cefiderocol, a siderophore cephalosporin able to overcome most resistance mechanisms, including metallo-beta-lactamases. Several studies are being carried to prove its clinical benefit. Case presentation: A 55-year-old male patient was admitted in the ICU undergoing septic shock due to surgical wound infection. Multidrug-resistant Pseudomonasputida grew in blood cultures and Pseudomonas aeruginosa grew in soft tissue cultures. He was treated with colistin and tobramycin, developing nephro and ototoxicity. Compassionate use of cefiderocol was ordered, and the infection was cured within 14 days. Conclusions: This is the first evidence of cefiderocol treatment in a soft tissue infection within a surgical wound infection. Our experience with cefiderocol in surgical wound infection suggests that it may be helpful in treating infections at that level, but more clinical trials are still needed.
Resumen
Antecedentes: Uno de los antibióticos más nuevos contra las bacterias multirresistentes (MDR) es el cefiderocol, una cefalos- porina siderófora capaz de superar la mayoría de los mecanismos de resistencia, incluidas las metalobetalactamasas. Se están realizando varios estudios para demostrar su beneficio clínico. Presentación del caso: Paciente masculino de 55 años que ingresó en la UCI con shock séptico por infección de herida quirúrgica. Pseudomonas putida multirresistente creció en hemocultivos y Pseudomonas aeruginosa crecieron en cultivos de tejidos blandos. Fue tratado con colistina y tobramicina, desarrollando nefro y ototoxicidad. Se indicó cefiderocol y la infección se curó en 14 días. Conclusiones: Esta es la primera evidencia de cefiderocol en el tratamiento de una infección de partes blandas dentro de una infección de herida quirúrgica. Nuestra experiencia con cefiderocol en infección de herida quirúrgica sugiere que puede ser útil en el tratamiento de infecciones a ese nivel, pero aún se necesitan más ensayos clínicos.
• MDR: Multi-drug Resistant.
• XDR: Extreme Drug Resistant.
• MBL: Metallo-beta-lactamase.
• VIM: Verona integron-encoded metallo—p—lactamase.
• PBP: Penicillin-binding Proteins.
• KPC: Klebsiella. pneumoniae carbapenemase.
seudomonas aeruginosa has one of the highest potential of producing antibiotic multi-resistance. According to European Centre for Disease Prevention and Control (ECDC), the proportion of Pseudomonas aeruginosa with combined resistance to at least three antibiotic families, or multidrugresistant bacteria (MDR) is 10,9% in 2018 in Spain. ENVIN-HELICS informed in 2018 about the isolation of 13,6% Pseudomonas aeruginosa in intra-ICU infections, with a 21% of antibiotic re- sistance[1].
The main mechanism for the development of intrinsic anti- biotic resistance is through constitutive production of inducible chromosomic AmpC p-lactamase and the production of cons- titutive or inducible efflux pumps[2],[3],[4]. It also has a large mutagenic potential, able to develop resistance to all antibiotics. Other inducible resistance mechanisms like AmpC hyper- production, MexAB-OprM or MexXY flow pumps, loss of OprD pores, spinning DNA mutations and topoisomerases[2],[3], could generate resistance to all antibiotics, even to the latest generation such as ceftazidime-avibactam and ceftolozan-tazo-bactam[3]. The selection of a mutant strain also facilitates the appearance of other resistance mechanisms.
Colistin remains a rescue antibiotic against MDR Gram-negative bacilli. However, it has adverse effects such as nephro and ototoxicity. Recent studies further suggest that it may be inferior as a treatment for new antibiotics[5].
Cefiderocol is a new drug with activity against carbapenem producing Gram-negative bacilli, including metallo-beta-lactamase (MBL). It is a siderophore cephalosporin with a chemical structure similar to ceftazidime and cefepime, which confers activity against Gram-negative microorganisms, and modifications that provide stability against hydrolysis by beta lactamases, better concentration in periplasmic space and the ability to use iron transport systems from the outer membrane of Gram-negative bacilli[6]. It is stable against hydrolysis by Ambler clas- ses A, C and D, and is the first drug stable against class B[7].
A 55-year-old male patient was admitted on a scheduled basis for segmental mandibulectomy and reconstruction with osteomyocutane locorregional pediculated flap of a previously necrosed pectoral flap. He was laryngectomized due to larynx cancer treated with chemotherapy, radiation therapy and surgery. Later he presented fistula, requiring reconstruction with pectoral. He has a history of smoking, enolic habit, dilated cardiomyopathy of non-ischemic origin with severe left ventricle dysfunction, carrier of cardiac resynchronizer, which needed replacement for Pseudomonas putida infection.
In the immediate postoperative period, it presents signs of congestive suffering in outer skin island of mandibular flap, purulent exudate and finally, it presents dehiscence of suture with necrosis of the cervical skin island. CT-scan was performed showing collection in submandibular and sublingual region, with probable hematic/infectious content. Empirical treatment is performed with meropenem and linezolid and they were dis- continued 48 hours later by isolation of Pseudomonas aeruginosa in exudate from surgical wound. He continued treatment with meropenem for 14 days with improvement of the surgical wound.
After 37 days of evolution, the patient had fever, chills and shivering. Pseudomonas putida positive for VIM-type MBL genes were isolated from blood culture, which was only sensitive to aminoglycosides and colistin, so treatment is changed to tobramycin and colistin. Subsequently, the patient presents unfavorable evolution with development of dizziness, instability and acute deterioration of renal function AKIN III. For that, colistin alone is left. On the 45th day the patient suffered acute respiratory deterioration that leads to cardiorespiratory arrest due to hypoxemia with spontaneous circulation recovery after 10 min of cardiopulmonary resuscitation. He was admitted to the ICU and required norepinephrine during the first few hours, and blood cultures were extracted. Antibiotic coverage is expanded with linezolid and meropenem. As the patient had exhibited vestibular and especially renal toxicity with tobramycin and colistin, and clearly these drugs were insufficient to treat the infection, compassionate use of cefiderocol was ordered. Previously, samples were taken from the surgical wound, where Pseudomonas aeruginosa was growing. In addition, the surgical wound was surgically cleaned. Progressive improvement of leukocytosis and procalcitonin is noted until the infection re- solution. Cefiderocol is withdrawn after 14 days of treatment. New swab from surgical wound was taken, in which no microorganism was growing. He is definitively discharged from UCI 4 days later.
In this report, we want to highlight how cefiderocol was effective in the treatment of a surgical wound infection by Pseudomonas spp. The patient had previously presented bacteremia for Pseudomonas putida positive for VIM-type MBL genes with serious adverse effects at the vestibular and renal level as a result of treatment with tobramycin and colistin, so we had very limited therapeutic options.
Cefiderocol therefore postulates as a safe and effective alternative. As far as we know, this is the first evidence of cefiderocol treatment in a soft tissue infection within a surgical wound infection. The doses were not individualized, and no data are available to determine its minimum inhibitory concentration, so clinical trials that outline the actual usefulness of cefiderocol for this type of infection are needed.
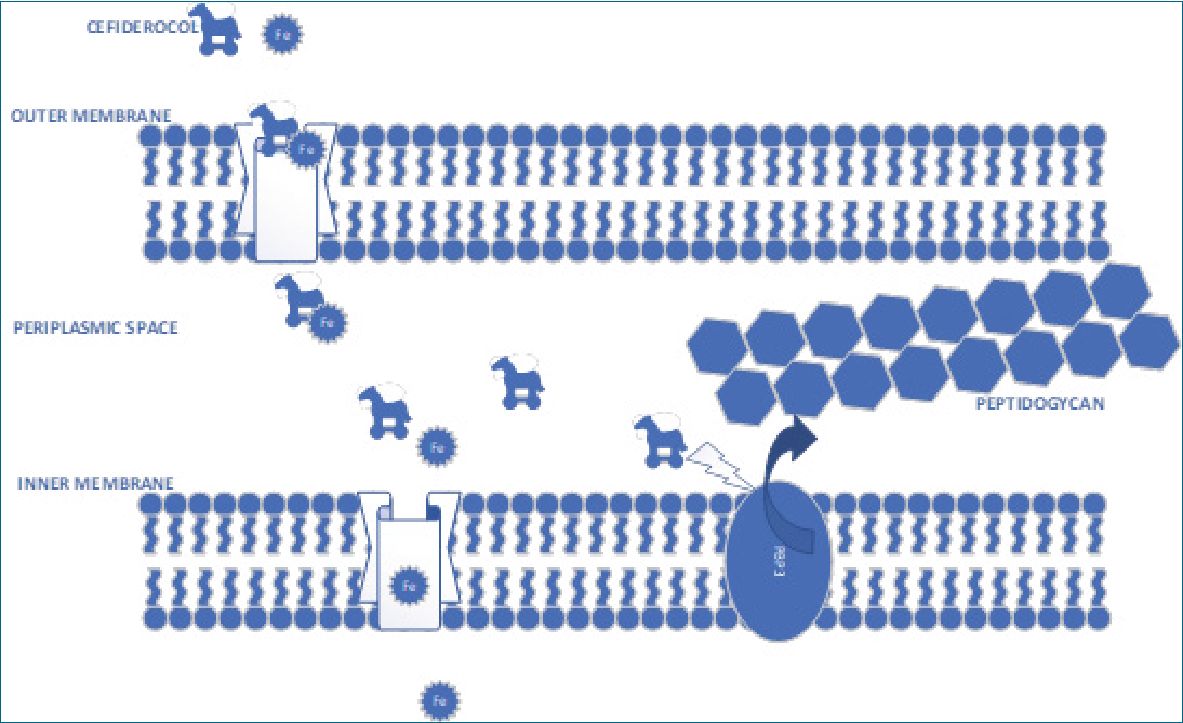
Figure 1.
The main feature of cefiderocol is its siderophore capacity. Siderophores are small molecules, with great iron chelating activity, which is produced by a multitude of bacteria and fungi. Cefiderocol forms complex with ferric iron and crosses the outer membrane, as if it were a Trojan horse (Figure 1), thanks to this siderophore property, even in the presence of porins and efflux pumps. This property is enhanced in inflammatory situations where extracellular iron is decreased. When it reaches periplasmic space, it binds to the penicillin-binding proteins (PBP3), inhibiting the synthesis of peptidoglycan and causing cell death like the rest of the cephalosporins, but with the advantage of its greater stability against almost all beta lactamases, including metallo-beta-lactamase[7]. This is relevant as, until now, there were few options against Ambler type B beta-lactamases except aztreonam.
There are to date two phase 3 and one phase 2 studies where cefiderocol is investigated. They are conducted by Shinogi Inc. Phase 2 APEKS-cUTI (ClinicalTrials.gov identifier NCT02321800) compares the efficacy and safety of cefiderocol versus imipenem/cilastatin in urinary tract infection (UTI). This is a multicenter, randomized, double-blind non-inferiority study in which cefiderocol 2g c/8 h is administered in one arm, and imipenecilastatin 1g c/8 h in patients with acute UTI with or without pyelonephritis by MDR Gram-negative pathogen. The result was non-inferiority in these patients[8].
CREDIBLE-CR phase 3 study (ClinicalTrials.gov identifier NTC02714595) studies the best therapy for severe infection by carbapenem-resistant Gram-negatives, including health care-associated pneumonia, primary bacteremia, nosocomial pneumonia, complicated urinary tract infection, sepsis and mechanical ventilation-associated pneumonia. In the experimental arm is given cefiderocol 2g c/8 h and in the control, usual antibiotherapy according to guidelines. APEKS-NP (ClinicalTrials. gov identifier NCT03032380) study compares mortality in patients with cefiderocol versus meropenem + linezolid in patients with nosocomial pneumonia, associated with health care and mechanical ventilation by Gram-negative pathogens.
Enrico Maria Trecarichi et al., report a case of sepsis by XDR Acinetobacter baumanii and Klebsiella pneumoniae producing KPC, which was successfully treated in cefiderocol monotherapy[9]. Also, Ryan W. Stevens et al. report success in treating an abdominal infection with MDR Pseudomonas aeruginosa using compassionate use of cefiderocol[10]. Jonathan D. Edgeworth even reports eradication of a MDR Pseudomonas aeruginosa based on an aortic valve[11]. In our case, although the multi- drug-resistant Pseudomonas putida was isolated in blood culture, our patient did have an infection of the surgical wound by a population of Pseudomonas aeruginosa, which was also
eliminated. This shows that cefiderocol is effective in soft tissue infections, being a location that needs to be studied in future works.
Cefiderocol is a well-tolerated drug with few adverse effects, which promises greater efficacy than other drugs for the treatment of multi- and pan-resistant Gram-negative microorganisms, especially in producers of MBL gene, as it is not inactivated by the usual resistance mechanisms. Although several studies are being carried out on its antimicrobial activity in urinary tract infection and in all types of pneumonia, its role in other types of infections has to be verified. Our experience with cefiderocol in surgical wound infection suggests that it may be helpful in treating infections at that level, but more clinical trials are still needed.
• Ethics approval and consent to participate: Not applicable.
• Consent for publication: Not applicable.
• Availability of data and materials: Not applicable.
• Competing interests: The authors declare that they have no competing interests.
• Funding: No funding was needed.
• Authors’ contributions: SGZ performed the literature re- search and made the diagram. CSC treated the patient and requested cefiderocol. CSC, SGP and RPS were major contributors in writing the manuscript. All authors read and approved the final manuscript.
• Acknowledgements: Not applicable.
References
1. Estudio nacional de vigilancia de infección nosocomial en servicios de medicina intensiva. ENVIN-HELICS, Barcelona. 1994. Accessed 02 Jan 2020.
2. Mensa J, Barberán J, Soriano A, Llinares P, Marco F, Cantón R, et al. Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa: Guidelines by the Spanish Society of Chemotherapy. Rev Esp Quimioter. 2018 Feb;31(1):78–100. PMID:29480677
3. Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, et al. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin Microbiol Rev. 2019 Aug;32(4):e00031-19. https://doi.org/10.1128/CMR.00031-19 PMID:31462403
4. del Barrio-Tofiño E, López-Causapé C, Cabot G, Rivera A, Benito N, Segura C, et al. Genomics and Susceptibility Profiles of Extensively Drug-Resistant Pseudomonas aeruginosa Isolates from Spain. Antimicrob Agents Chemother. 2017;61(11):e01589-17, /aac/61/11/e01589-17.atom. https://doi.org/10.1128/AAC.01589-17.
5. van Duin D, Lok JJ, Earley M, Cober E, Richter SS, Perez F, et al.; Antibacterial Resistance Leadership Group. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin Infect Dis. 2018 Jan;66(2):163–71. https://doi.org/10.1093/cid/cix783 PMID:29020404
6. Karlowsky JA, Kazmierczak KM, de Jonge BL, Hackel MA, Sahm DF, Bradford PA. In Vitro Activity of Aztreonam-Avibactam against Enterobacteriaceae and Pseudomonas aeruginosa Isolated by Clinical Laboratories in 40 Countries from 2012 to 2015. Antimicrob Agents Chemother. 2017 Aug;61(9):e00472-17. https://doi.org/10.1128/AAC.00472-17 PMID:28630192
7. Zhanel GG, Golden AR, Zelenitsky S, Wiebe K, Lawrence CK, Adam HJ, et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs. 2019 Feb;79(3):271–89. https://doi.org/10.1007/s40265-019-1055-2 PMID:30712199
8. Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JC, Ariyasu M, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018 Dec;18(12):1319–28. https://doi.org/10.1016/S1473-3099(18)30554-1 PMID:30509675
9. Trecarichi EM, Quirino A, Scaglione V, Longhini F, Garofalo E, Bruni A, et al.; IMAGES Group. Successful treatment with cefiderocol for compassionate use in a critically ill patient with XDR Acinetobacter baumannii and KPC-producing Klebsiella pneumoniae: a case report. J Antimicrob Chemother. 2019 Nov;74(11):3399–401. https://doi.org/10.1093/jac/dkz318 PMID:31369095
10. Stevens RW, Clancy M. Compassionate Use of Cefiderocol in the Treatment of an Intraabdominal Infection Due to Multidrug-Resistant Pseudomonas aeruginosa: A Case Report. Pharmacotherapy. 2019 Nov;39(11):1113–8. https://doi.org/10.1002/phar.2334 PMID:31550054
11. Edgeworth JD, Merante D, Patel S, Young C, Jones P, Vithlani S, et al. Compassionate Use of Cefiderocol as Adjunctive Treatment of Native Aortic Valve Endocarditis Due to Extremely Drug-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2019 May;68(11):1932–4. https://doi.org/10.1093/cid/ciy963 PMID:30418554

 ORCID
ORCID