Ismail Mohammed Ibrahim MD.1,*, Maha Sadek Elderh MD.1, Tamer Samir Abdelsalam MD.1
Recibido: 03-12-2021
Aceptado: 28-12-2021
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 1 pp. 73-82|https://doi.org/10.25237/revchilanestv5208111147
PDF|ePub|RIS
Adición de dexmedetomidina a bloqueo compartimental de fascia ilíaca endovenosa en artroscopia de cadera: Estudio randomizado
Abstract
Stable hemodynamics in hip arthroscopy is one of the most important tasks. Recently, dexmedetomidine has been considered a safe analgesic and sedative adjuvant in different surgeries. Ultrasound-guided fascia iliaca compartment block (FICB) is a technique used to produce postoperative analgesia for hip surgery. FICB will be a promising technique for postoperative analgesia in hip arthroscopy. We designed a prospective, randomized, double-blind, parallel-group trial to evaluate the effect of administration of dexmedetomidine intravenously and perineurally on hemodynamic stability intraoperatively and on narcotics consumption postoperatively, as well as to evaluate the effect of dexmedetomidine as an adjuvant to FICB. A total of 88 patients scheduled for elective hip arthroscopy, aged 18 to 65 years, 70-80 kg, both sexes. Patients were randomized into Group A (intravenous Dexmedetomidine) and Group B (perineural Dexmedetomidine). The Main outcome measure was to detect effectiveness of dexmedetomidine infusion without loading dose in achieving intraoperative hemodynamic stability with minimal need for rescue agents and its analgesic effect postoperatively, if added to bupivacaine in fascia iliaca block. Dexmedetomidine significantly maintained mean arterial blood pressure and heart rate (P-value <0.001); with lower intraoperative fentanyl consumption in group A (53.85 ± 13.87 pg) when compared to group B (150.00 ± 40.35 pg) and no significant difference between the two groups in VAS scores postoperatively. Dexmedetomidine infusion provided stable intraoperative hemodynamics in patients undergoing hip arthroscopy with reduced intraoperative narcotics requirement, while addition of dexmedetomidine to bupivacaine had no role in prolonging the effect of FICB.
Resumen
La estabilidad hemodinamica en la artroscopia de cadera es una de las tareas más importantes. Recientemente, la dexmedetomidina se ha considerado un coadyuvante analgésico y sedante seguro en diferentes cirugías. El bloqueo compartimental de la fascia ilíaca guiado por ultrasonido (FICB) es una técnica utilizada para producir analgesia posoperatoria para la cirugía de cadera. FICB podría ser una técnica prometedora para la analgesia posoperatoria en artroscopia de cadera. Se designa un protocolo prospectivo randomizado doble ciego y paralelo para evaluar el efecto hemodinámico intraoperatoria de la administarción de dexmedetomidina endovenosa (EV) y perineural. Además se evaluó el uso de narcóticps posoperatorios como el efecto de la dexmedetomidina como adjuvante de FICB. Un total de 88 pacientes entre 18 y 65 años entre 70 – 80 kg, y de ambos sexos fueron enrolados en dos grupos grupo A (Dexmedetomidina EV) y grupo B (Dexmedetomidina perineural). El objetivo principal fue evaluar la efectividad de la infusión de dexmedetomidina sin dosis de carga en lograr una adecuada estabilidad hemodinámica y evaluar en el posoperatorio la necesidad de analgesia de rescate (Grupo A). En el grupo B se evaluó si la adición de dexmedetomidina a la bupivacaina infiltrada en la fascia de manera local, mejora la analgesia. La dexmedetomidine mantiene de manera significativa la PAM y frecuencia cardíaca (P-value < 0,001); con bajo consumo de fentanyl intraoperatorio en el groupo A (53,85 ± 13,87 pg) al compararlo con el grupo B (150,00 ± 40,35 pg) y no se encontraron diferencias en el dolor posoperatorio entre ambos grupos. La infusión de dexmedetomidina permite una hemodinamia intraoperatoria estable en pacientes sometidos a artroscopía de cadera con un bajo requerimiento de narcóticos. Mientras que la adición de dexmedetomidina al bloqueo de la fascia realizado con bupivacaina, no tiene efecto en prolongar su efecto.
-
Introduction
During the last decade, hip arthroscopy has become a common surgery; with numbers of cases increasing rap- idly all over the world. More than 11,000 hip arthroscopies were operated at the English public health system from 2002 to 2013, showing more than 700% increase. Similarly, in North America, there was an increase of more than 350% in the period from 2004-2009. On the opposite side of the globe, in Korea, there was only a twofold increase between 2007 and 2010, with an expected increase of 13.88% by 2023[1].
Fascia iliaca compartment block (FICB) was first described by Dalens et al. on children using a landmark technique. It is a simple, easy to do and low-cost technique which provides an efficient and adequate perioperative analgesia for patients suffering from hip or femur fractures. The procedure has become easier, with higher success rates because of the use of ultra- sound during the block, which is commonly found in operating theatres and emergency rooms. Indications for FICB include pre-, peri- and postoperative analgesia after fractured neck of the femur. Additional indications include hip and knee surgery, above knee amputation, and application of plaster cast to femoral fracture in pediatric patients, although data to support these indications are limited[2]. The fascia iliaca block is also called the fascia iliaca compartment block, which is considered an alternative to a femoral nerve or a lumbar plexus block[3].
Fascia iliaca compartment block can be conducted in many ways, either as a single shot or a continuous infusion. The technique of continuous infusion catheter is similar to that of a single shot in which a bolus dose of local anesthetic (LA) is injected followed by the intermittent technique, either a con- tinuous infusion and/or intermittent boluses of LA through an infusion pump. Infusion pumps are electronic or non-electronic; e.g., elastomeric pumps[4].
During patient positioning for spinal anesthesia in femur fracture surgeries, FICB provides better analgesia compared to IV opioids[5], where the fascia iliaca compartment catheter can be placed faster than femoral nerve catheter, but with retarded onset of sensory block[6].
Bupivacaine, an amide local anesthetic drug, is an abundant and low-price drug; however, it possesses a delayed onset of action. Dexmedetomidine is an adjuvant that can help solve this problem[7].
Dexmedetomidine is an a2 adrenergic receptor agonist that has analgesic and sedative effects, but with no respiratory depression by having a2 receptor affinity. Li et al., suggested that dexmedetomidine can elevate the quality and prolong the duration of local anesthetic blockade. Addition of dexmedeto- midine to bupivacaine helped to enhance the onset time for sensory block during spinal, epidural, and brachial plexus blocks with good results[8].
Dexmedetomidine also has an amnestic action, decreases postoperative pain intensity, has opioid-sparing effects and less postoperative nausea and vomiting that help fast track recovery in various types of surgeries. It is considered a useful and safe adjunct to general anaesthesia for various surgical procedures [9].
The current study aims to assess the efficacy of dexmedetomidine as an adjuvant to general anesthesia on hemodynamics and intraoperative narcotic consumption as a primary outcome. It further assesses its effect as an adjuvant to FICB when added to bupivacaine in the postoperative period, regarding analgesia after hip arthroscopy as a secondary outcome.
We designed this randomized double-blinded study to evaluate the effectiveness of dexmedetomidine intravenously versus perineurally in FICB during hip arthroscopy under GA. We compared intraoperative hemodynamics and perioperative narcotic consumption. We hypothesize that dexmedetomidine added intravenously will have a favorable effect on hemodynamic and will prolong the effect of FICB, when added perineurally.
-
Materials and Methods
This study was approved by the Ethics Committee, Faculty of Medicine, Ain Shams University, Cairo, Egypt (FMASU R116/2021; May 17, 2021), and it was conducted in accordance with the principles of the Declaration of Helsinki. An informed written consent was obtained from each patient. Confidentiality of the data was maintained by assigning a code number to each patient. The trial was registered at ClinicalTri- als.gov (NCT04917029; June 8, 2021).
We intend to share the individual didentified participants’ data. Data will be accessible through direct contact with the corresponding author, beginning 12 months and ending 36 months following publication.
We enrolled adult patients (18 to 65 years old) scheduled for elective hip arthroscopy under general anesthesia, who were from the American Society of Anesthesiology (ASA) I or II and had body weight of 70 – 80 kgs. We excluded obese and addict patients, patients who were on anticoagulants or chronic pain medications and those who had coagulation defects, local infection or previous surgery at the injection site, mental or psychiatric disorders, impaired liver or kidney functions, peripheral neuropathy, or bronchial asthma. In addition, we excluded patients who were allergic to any of the study drugs and those who refused to participate.
Patients undergoing hip arthroscopy under general anesthesia were randomly assigned in this study into one of the following two groups:
Group A: (44 patients) received 40 ml bupivacaine 0.25% +5 ml normal saline in FICB and 0.5 pg/kg/h of dexmedetomidine intravenous infusion during general anesthesia.
Group B: (44 patients) received dexmedetomidine 80pg diluted in 5 ml normal saline and 40 ml bupivacaine 0.25% and were anesthetized generally without dexmedetomidine infusion (with normal saline infusion parenterally instead of dexmedetomidine).
We used the sealed, opaque, sequentially-numbered envelopes method for randomization and allocation concealment. We prepared 44 identical, opaque, letter-sized envelopes; each containing a white allocation paper (Marked as “Treatment A”) and a sheet of single-sided carbon paper closest to the front of the envelope (with the carbon side facing the white paper). Finally, the envelopes were sealed, and we signed across the seal. Likewise, we prepared 44 “Treatment B” envelopes. The two sets (88 envelopes) were combined and shuffled thoroughly. Using a pen, we marked a number on the front of each envelope sequentially from 1 to 88. Then, we placed these envelopes into a plastic container, in numerical order, ready for use. An investigator (not involved in sequence generation and al- location concealment) assessed patients for eligibility and as- signed eligible patients to either dexmedetomidine intravenous infusion (Group A) or dexmedetomidine local injection (Group B). Participants, care providers, outcomes assessors, and data analysts were ignorant of the treatment allocation.
After admission to the operating room, preanesthetic medication was given in the form of intravenous (IV) midazolam (0.03 mg/kg) and Granisetron (1 mg) for prophylaxis against nausea and vomiting, simultaneously with monitoring (electro- cardiogram, non-invasive blood pressure monitoring and oxy- gen saturation [SpO2]).
Preoxygenation was started for three minutes, and anaesthesia was conducted by administration of propofol (2-3 mg/ kg), fentanyl (1 pg/kg), and atracurium besylate (0.5 mg/kg), followed by endotracheal intubation. Maintenance of anesthesia was attained by mechanical ventilation with a tidal volume of 8 ml/kg, 1-2 minimum alveolar concentration of isoflurane mixed with oxygen (50%) and air (50%) were used.
Group A received dexmedetomidine infusion in a dose of 0.5 pg/kg/hour, it stimulates a biphasic response of blood pressure: A short initial hypertensive period followed by hypotension. It is found that hypotension and bradycardia will happen with ongoing therapy within 15 minutes, due to its effect on central a2 adrenergic receptor agonist which decreases the release of noradrenaline from the sympathetic nervous system [10], that is why we started dexmedetomidine infusion just after intubation.
After sterilization using povidone iodine, ultrasound-guided block was performed in supine position. First by identifying inguinal ligament, a 7.5 – 12 MHz linear probe was used on the inguinal crease parallel to the inguinal ligament to identify femoral artery, with a little movement of the probe laterally till the iliopsoas muscle appears as a hypoechoic part in lateral to the artery and femoral nerve. In-plane technique was per- formed by a 22G/80 mm insulated echogenic needle, in which femoral nerve that is covered by a hyperechoic fascia and found between the muscle and the subcutaneous tissue needle was directed and advanced towards the fascia iliaca and iliopsoas muscle. To confirm correct passage of the needle through the fascia iliaca, fascial click and 2 mL of saline were used. Local an- esthetic injected between fascia iliaca and iliopsoas muscle was visualized by hydro dissection technique shown on ultrasound, through which the needle moved in the space created by the injected LA. The anesthesiologist in charge was aware of the nature of drug in each syringe, and the operation was attended by personnel who did not participate in the study.
Group B received dexmedetomidine (80 pg diluted in 5 ml of normal saline) and 40 ml of bupivacaine 0.25% and were anesthetized generally with no dexmedetomidine infusion (with normal saline infusion parenterally instead of dexmedetomidine).
Continuous monitoring of the mean arterial pressure (MAP) and the heart rate (HR) was done. Any change in the average data was managed promptly after exclusion of a surgical cause. Increase of either the MAP or the HR 20% above the baseline was treated by 0.5 pg/kg of IV fentanyl.
Decline of mean arterial blood pressure more than 20% and reduction of isoflurane concentration to 0.6% were noted. If the patient was still hypotensive, 6 mg of ephedrine was given intravenously. Finally, bradycardia was treated with 0.6 mg of IV atropine and repeated as required.
Increments of fentanyl (0.5 pg/kg IV) were administrated intraoperatively but stopped one hour prior to the end of surgery and no other intraoperative analgesic adjuncts were given.
At the end of the procedure, neuromuscular blockade was reversed with neostigmine (0.05 mg/kg) and atropine (0.02 mg/ kg). Patients were extubated after they attained the ability to obey simple commands, then transferred to PACU, where 0 h postoperatively was defined and started.
The primary outcome was detecting the effectiveness of dexmedetomidine infusion without loading dose in achieving intraoperative hemodynamic stability with minimal need of res- cue agents (which was detected by measuring the frequency and the total amount of fentanyl needed intraoperatively). Tepid fluctuations in heart rate or mean arterial blood pressure by values less than 20% after surgical stimuli were considered as a good and stable hemodynamic applied anaesthetic technique.
The secondary outcome was assessed postoperatively by the Visual Analogue Scale (VAS), which is a subjective measure for acute and chronic pain. The VAS was recorded at 2, 3, 4, 6, 8, 12, 18, and 24 h. A fixed dose of 1 g of intravenous acetaminophen was given/8 h. If VAS pain score was more than 3 or the patient asked for analgesia, IV pethidine (20 mg) was administered as rescue analgesic and could be repeated after 20 minutes till VAS < 3. The time of the first analgesic request and the total 24 h analgesic requirement were recorded and compared.
Sample size was calculated using the G*power 3.1.9.2 software. The alpha error level was set at 0.05, and the power was set at 0.80. The effect size was calculated based on earlier re- ports[11],[12] assuming a medium effect size difference of 0.3 in intraoperative hemodynamic stability and postoperative analgesia between the two groups (adding dexmedetomidine perineurally to bupivacaine in ultrasound-guided fascia iliaca block and intravenously infused dexmedetomidine) in patients under- going hip arthroscopy. Based on this assumption, a sample size of at least 88 patients planning hip arthroscopy (44 patients per group) was sufficient to achieve the study objectives.
Data was interpreted and analysed using SPSS v.25 (IBM©, Chicago, IL, USA). Quantitative parametric data were presented as mean and standard deviation and was analysed by unpaired student t-test. Quantitative non-parametric data was presented as median and interquartile range and were analysed by Mann Whitney-test. Qualitative data was presented as number and percentage, and Chi-square (X2) or Fisher’s Exact tests were used, when appropriate, to compare this data. P value < 0.05 was considered statistically significant.
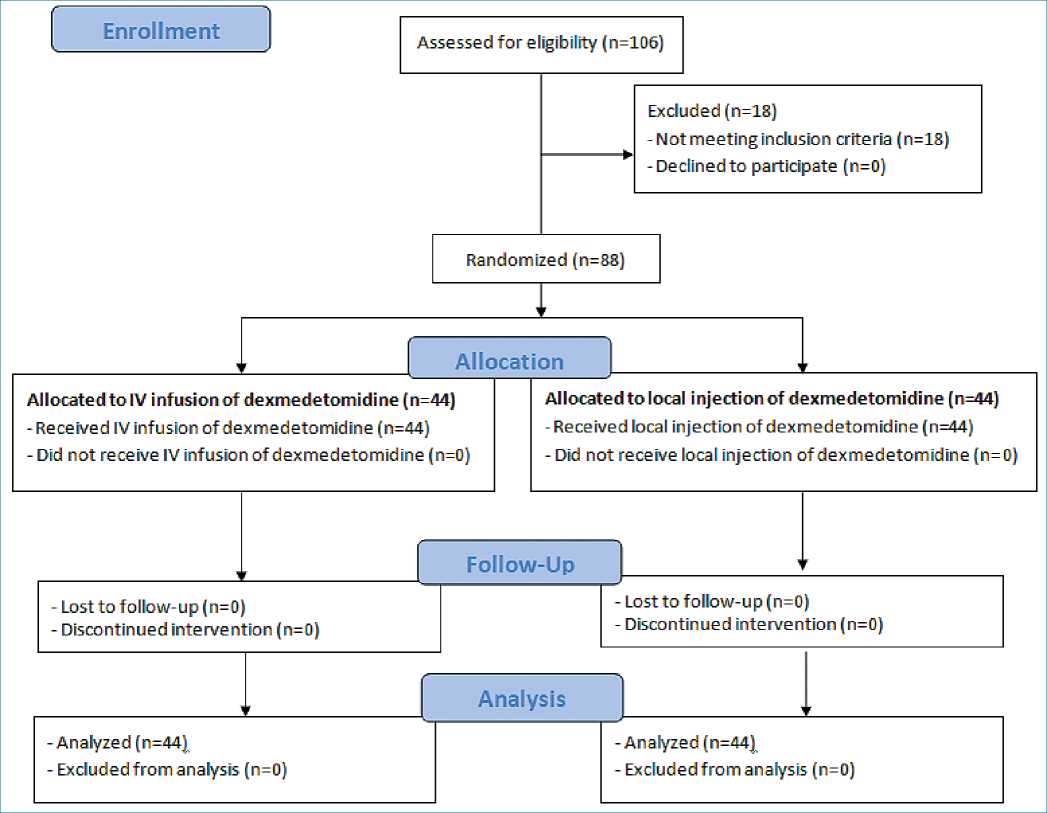
Figure 1. The trial CONSORT flowchart.
-
Results
One hundred and six patients scheduled for elective hip arthroscopy under general anesthes ia were screened for eligibility. 88 of them met the eligibility criteria and were randomly allocated to receive either dexmedetomidine by IV infusion (n = 44) or dexmedetomidine by local injection (n = 44) (Figure 1)
We found no significant differences between the two groups in terms of age, gender, and body weight (Table 1).
All the surgical procedures were completed without complications.
No significant differences were observed regarding the hemodynamic parameters at the baseline readings and after intubation. Although patients in group A with dexmedetomidine infusion did not have a significant rise in MAP (Table 2) together with lower HR (as shown in (Table 3) during surgery group B had a significant rise in MAP and HR as shown in Figures 2, 3 and Figure 4 show significantly higher mean intraoperative additional fentanyl requirements in group B.
There was no significant difference between the two groups regarding VAS scores measured during the first 24 h postoperatively (Table 5).
-
Discussion
In our study, none of the patients were excluded and both groups were statistically comparable with respect to age, body weight and gender. We conclude that the use of dexmedetomidine perioperatively with no loading dose for hip arthroplasty provided a stable intraoperative hemodynamics. Dexmedetomidine infusion induces an initial transient elevation in MAP (due to activation of postsynaptic 2B receptors), followed by a decline in MAP and HR (by the activation of 2A receptors in the central nervous system). Ceasing dexmedetomidine loading bolus can prevent initial hypertension[13].
Table 1. Difference between 2 groups regarding age, gender, and body weight
| Variable | Group A n = 44 | Group B n = 44 | Test value | P-value |
| Age (years) Mean ± SD
Range |
37.23 ± 12.58
18 – 64 |
38.25 ± 11.98
18 – 65 |
-0.390» | 0.697 |
| Body weight (kg) Mean ± SD
Range |
75.80 ± 2.78
70 – 80 |
75.98 ± 2.71
70 – 80 |
-0.311» | 0.757 |
| Gender Female
Male |
22 (50.0%)
22 (50.0%) |
22 (50.0%)
22 (50.0%) |
0.000# | 1.000 |
Table 2. Comparison between the two groups regarding intraoperative mean arterial pressure monitoring
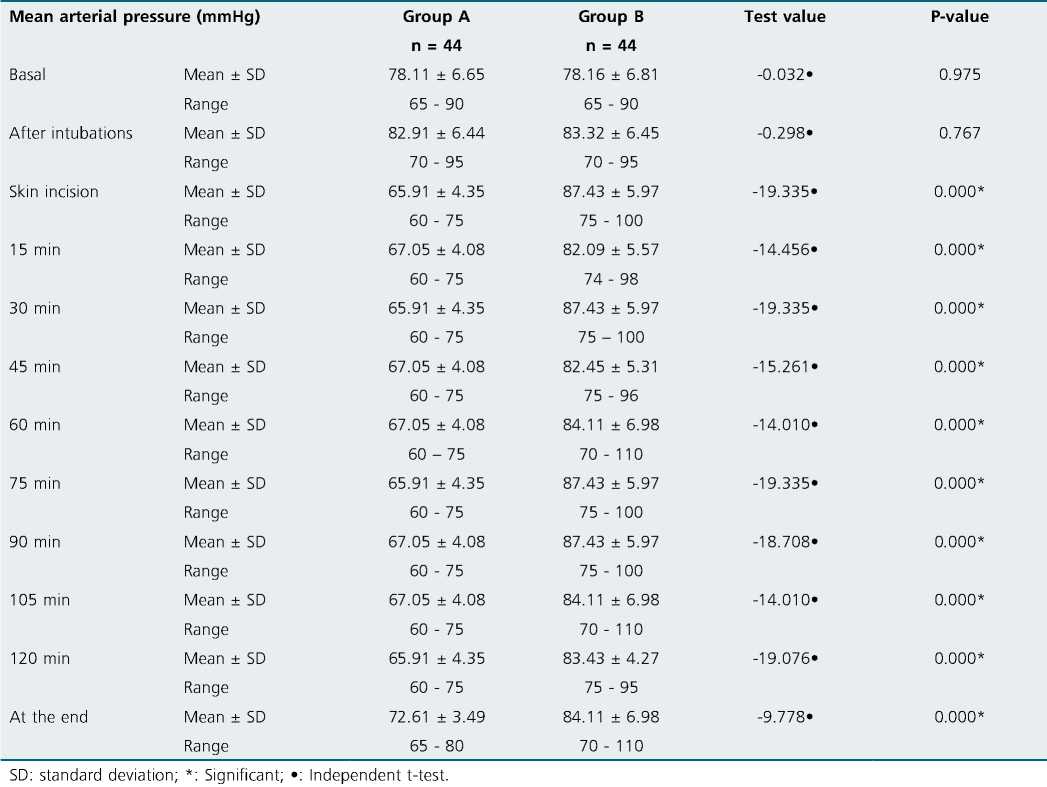
Dexmedetomidine infusion established intraoperative hemodynamic stability, and this finding is supported by many studies[14]-[16]. Attenuated intraoperative hemodynamic stress response can be achieved, whether given as 0.4 pg/kg/h without loading dose[17] or in a ranging dose of 0.4 to 0.6 pg/ kg/h after the loading dose of 1 pg/kg/h[11],[16],[18].
This finding was also achieved when administering dexmedetomidine in a target-controlled infusion[19] or in a high maintenance dose[15]. Additionally, with the use of dexmedetomidine, there was improvement of the hemodynamic stability in patients with bispectral index (BIS)-guided anesthesia[5],[18],[20]. In this study, patients didn’t suffer from significant bradycardia requiring intervention or hypotension because of the low infusion dose of dexmedetomidine used. Dexmedetomidine is known to have an analgesic property[21]. Consistent with this study, we recorded significantly lower intraoperative requirements of analgesics.
Table 3. Comparison between the two groups regarding intraoperative heart rate monitoring
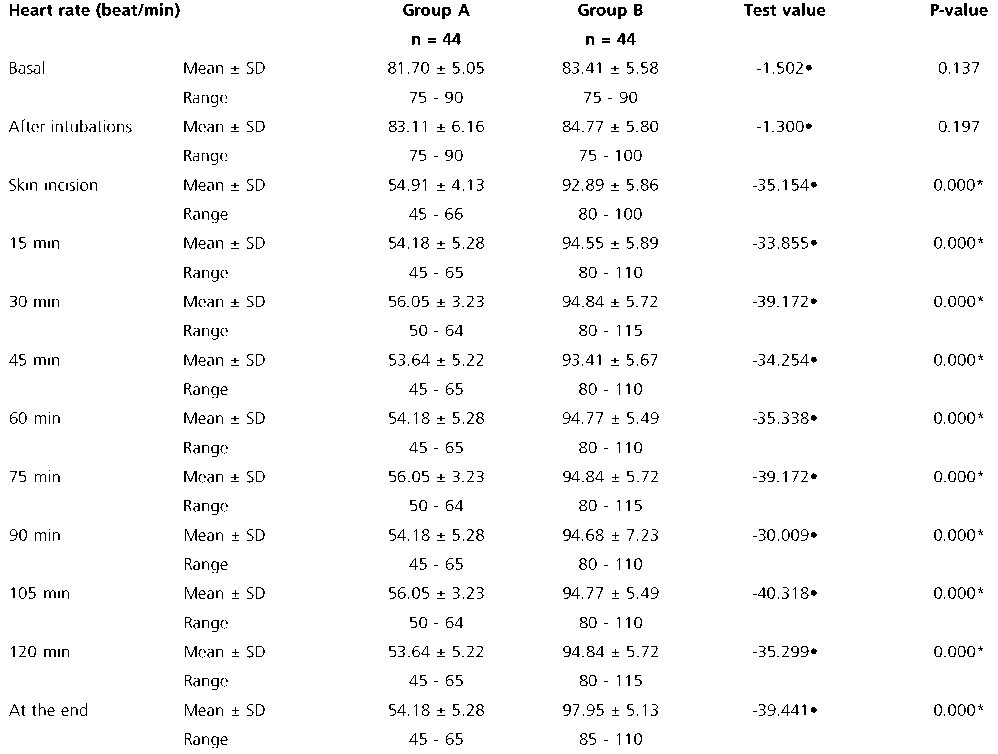
SD: standard deviation; *: Significant; •: Independent t-test.
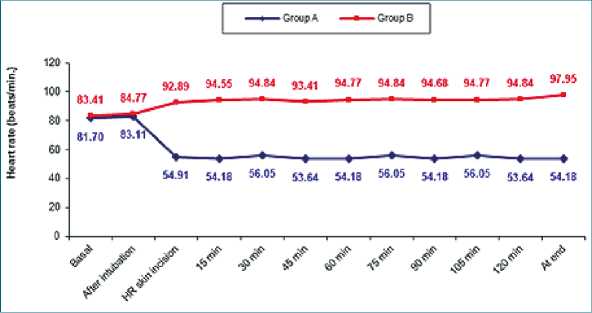
Figure 2. Comparison between two groups in intraoperative heart rate monitoring
Anesthetic and analgesic sparing effects of dexmedetomidine during surgeries were concluded in many stud- ies[11],[18]-[20] that have come along our findings. For ex- ample, Chakrabati et al.[22] noticed a significant reduction of intraoperative -BIS guided-fentanyl and propofol utilization in patients undergoing cerebellopontine angle surgeries. Different studies done on other types of surgeries confirmed the same finding[23],[24].
In contrast to our findings, Sriganesh and their colleagues[25],[26] demonstrated that dexmedetomidine infusion of 0.5 pg/kg/h with no loading dose didn’t have a more favorable effect than fentanyl. This result could have been affected by the bilateral scalp block given in their patients, which may have influenced intraoperative surgical stress response to the study drugs independently, thus abolishing hemodynamic differences between both groups. Moreover, Rajan et al.[27] ob- served the same findings during brain tumor surgery, in which dexmedetomidine infusión of 0.5-1 pg/kg/h after a loading dose didn’t reveal superiority over remifentanil infusion. This could have been attributed to the high potency of remifentanil’s analgesic properties.
Fascia iliaca block is a safe and effective approach for pain management[28] that has proved to be more effective than femoral nerve blocks in alleviating postoperative pain after hip surgeries[29],[30].
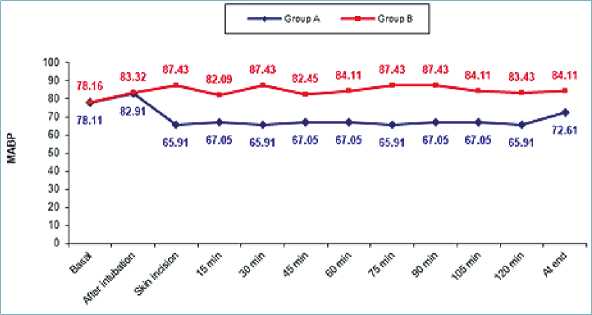
Figure 3. Comparison between two groups in intraoperative mean blood pressure (MBP) monitoring.
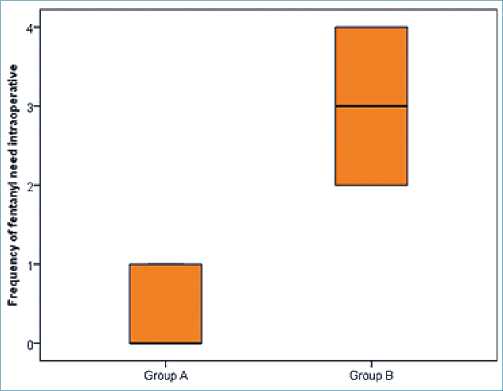
Figure 4. Comparison between two groups in the frequency of fentanyl need intraoperative.
Bupivacaine as a local anesthetic has been used in various studies, with different degrees of efficacy due to the different concentrations administrated. Some studies have shown that usage of 0.5% bupivacaine for peripheral nerve blocks provides longer duration of postoperative analgesia for lower limb sur- geries than 0.25% bupivacaine. However, another study has shown that either 0.5% bupivacaine or 0.25% was accompanied by adequate analgesia with no difference in the duration of action, even though patients receiving 0.5% bupivacaine had lower satisfaction due to the occurrence of numbness, weakness, and delay in walking[7].
A growing attention is directed to choosing the LA adjuvants that accelerate the onset of action and prolong the analgesic effect of LA in FICB[31],[32]. Dexmedetomidine, used as an adjunct to bupivacaine for FICB in our study, is a highly selective central alpha-2 agonist and has sedative, anxiolytic and analgesic properties[33]. Intravenous infusion of dexmedetomidine has been shown to decrease pain scores when used in conjunction with FICB[34].
In dis-concordance to our results, multiple studies have shown that using dexmedetomidine as an adjuvant to LA in peripheral nerve techniques enhances the onset of action, increases the duration of analgesia time to first rescue analgesia with improved quality of block and decreased postoperative analgesic intake[35],[36].
Table 4. Comparison between the two groups regarding the frequency and total amount of fentanyl need intraoperative
| Variable | Group A n = 44 | Group B n = 44 | Test value | P-value |
| Frequency of fentanyl Median (IQR) | 0 (0 – 1) | 3 (2 – 4) | 8.336- | 0.000* |
| need intraoperative Range | 0 – 1 | 2 – 4 | ||
| Total amount of Mean ± SD | 53.85 ± 13.87 | 150.00 ± 40.35 | -8.401» | 0.000* |
| fentanyl need Range intraoperative | 50 – 100 | 100 – 200 | ||
| IQR: interquartile range; SD: standard deviation; *: Significant; •: Independent t-test; *: Mann-Whitney test. | ||||
Regarding FICB, contrary to our results, Sabra et al.[37] added dexmedetomidine to local anesthetic in order to study its postoperative analgesic effect in hip arthroplasty. They re- ported that combining dexmedetomidine with local anesthetic significantly decreases pain scores and analgesic hours’ requirement, as opposed to solely using ropivacaine.
Sivakumar et al.[32] also added dexmedetomidine to LA in FICB for femur surgeries, but with general anesthesia, while Li et al. [38] studied the effect of adding dexmedetomidine to LA in FICB for knee arthroscopy. Both studies proved the decrease of pain scores and the total analgesic requirement. To be noted, in these studies, all injections were given after anesthesia. Hence, the time of analgesia onset could not be assessed. Mechanism of action of dexmedetomidine on peripheral nerves is not yet clear. Some suggest its action through direct drug effect on the nerves and absorption into systemic circulation causing central effect [32]. No adverse effects were recorded in our patients. On the other hand, FICB can reduce narcotic- related side effects, and this was shown in many studies [39]. The VAS scores were similar in both groups in the first 24 hours, and no rescue analgesia was required in group A&B in the first 24 hours postoperatively, thus indicating that dexmedetomidine has no role in the reduction in analgesic requirement. In dis-concordance to our results, El-Rahmawy and Hayes [40] observed that time for administration of rescue was significantly later in the patients who received dexmedetomidine in local anesthesia for FICB. In addition, dexmedetomidine group had significantly lower VAS scores and decreased rescue analgesic consumption in the dexmedetomidine group.
-
Limitations
The limitation of our study is that the patients were operated on by different surgeons, thus introducing possibilities of differences in tissue handling and the resulting pain. Further dose-response studies may be planned to find out an optimal dose for use as an adjuvant in FICB. There was no long-term follow up, as hospital stay and long-term neurological complications were not assessed.
Table 5. Comparison between the two groups regarding the Visual Analogue Scale scores during the first 24 hours postoperatively
| Visual Analogue Scale score | Group A n = 44 | Group B n = 44 | Test value | P-value | |
| 2 h | Median (IQR)
Range |
0(0 – 1)
0 – 1 |
0 (0 – 1)
0 – 1 |
-1.109* | 0.267 |
| 3 h | Median (IQR)
Range |
0(0 – 1)
0 – 1 |
0 (0 – 1)
0 – 1 |
-1.109* | 0.267 |
| 4 h | Median (IQR)
Range |
0(0 – 1)
0 – 1 |
0 (0 – 1)
0 – 1 |
-1.109* | 0.267 |
| 6 h | Median (IQR)
Range |
0(0 – 1)
0 – 1 |
0 (0 – 1)
0 – 2 |
-0.565* | 0.572 |
| 8 h | Median (IQR)
Range |
0 (0 – 1)
0 – 1 |
0 (0 – 1)
0 – 1 |
-0.657* | 0.511 |
| 12 h | Median (IQR)
Range |
0(0 – 1)
0 – 1 |
0 (0 – 1)
0 – 2 |
-0.565* | 0.572 |
| 18 h | Median (IQR)
Range |
2 (1 – 2)
0 – 2 |
2 (1 — 2)
1 – 2 |
-0.948* | 0.343 |
| 24 h | Median (IQR)
Range |
2 (2 – 3)
0 – 3 |
2 (2 – 3)
1 – 3 |
-0.455* | 0.649 |
-
Conclusion
Infusion of dexmedetomidine at a dose of 0.5 g/kg/h with no loading dose provided a stable intraoperative hemodynamics in patients undergoing hip arthroscopy. The results of the present study suggest that the addition of dexmedetomidine to bupivacaine has no role in prolonging the effect of FICB in these patients and no significant adverse effects.
Trial Registration: (NCT04917029; June 8, 2021).
References
1. Maradit Kremers H, Schilz SR, Van Houten HK, Herrin J, Koenig KM, Bozic KJ, et al. Trends in Utilization and Outcomes of Hip Arthroscopy in the United States Between 2005 and 2013. J Arthroplasty. 2017 Mar;32(3):750–5. https://doi.org/10.1016/j.arth.2016.09.004 PMID:27793498
2. O’Reilly N, Desmet M, Kearns R. Fascia iliaca compartment block. BJA Educ. 2019 Jun;19(6):191–7. https://doi.org/10.1016/j.bjae.2019.03.001 PMID:33456890
3. Hadzic A. Hadzic’s textbook of regional anesthesia and acute pain management. Secon Edition ed: McGraw-Hill; 2017.
4. Scala VA, Lee LS, Atkinson RE. Implementing Regional Nerve Blocks in Hip Fracture Programs: A Review of Regional Nerve Blocks, Protocols in the Literature, and the Current Protocol at The Queen’s Medical Center in Honolulu, HI. Hawaii J Health Soc Welf. 2019 Nov;78(11 Suppl 2):11–5. PMID:31773105
5. Madabushi R, Rajappa GC, Thammanna PP, Iyer SS. Fascia iliaca block vs intravenous fentanyl as an analgesic technique before positioning for spinal anesthesia in patients undergoing surgery for femur fractures-a randomized trial. J Clin Anesth. 2016 Dec;35:398–403. https://doi.org/10.1016/j.jclinane.2016.09.014 PMID:27871563
6. Plečko M, Bohaček I, Tripković B, Čimić M, Jelić M, Delimar D. Applications and critical evaluation of fascia iliaca compartment block and quadratus lumborum block for orthopedic procedures. Acta Clin Croat. 2019 Jun;58 Suppl 1:108–13. https://doi.org/10.20471/acc.2019.58.s1.16 PMID:31741568
7. Rahimzadeh P, Imani F, Sayarifard A, Sayarifard S, Faiz SH. Ultrasound-guided fascia iliaca compartment block in orthopedic fractures: Bupivacaine 0.2% or 0.3%? Med J Islam Repub Iran. 2016 Oct;30:433. PMID:28210598
8. Abotaleb U, Abdalla A, Abdelrahman A, Gad G, Elsayed A. Dexmedetomidine versus granisetron for the management of postspinal shivering. Ain-Shams Journal of Anaesthesiology. 2016;9(4):517–23. https://doi.org/10.4103/1687-7934.198259.
9. Wang L, Shen J, Ge L, Arango MF, Tang X, Moodie J, et al. Dexmedetomidine for craniotomy under general anesthesia: A systematic review and meta-analysis of randomized clinical trials. J Clin Anesth. 2019 May;54:114–25. https://doi.org/10.1016/j.jclinane.2018.11.001 PMID:30445412
10. Moharram AA, Mostafa RH. Efficacy of Dexmedetomidine Infusion Without Loading Dose as a Potent Hypotensive Agent in Lumbar Fixation Surgery. The Open Anesthesia Journal. 2019;13(1):68–74. https://doi.org/10.2174/2589645801913010068.
11. Ilhan O, Koruk S, Serin G, Erkutlu I, Oner U. Dexmedetomidine in the supratentorial craniotomy. Eurasian J Med. 2010 Aug;42(2):61–5. https://doi.org/10.5152/eajm.2010.19 PMID:25610125
12. Amin M, Abdalla A, Soltan S. Efficacy of adding Dexmedetomidine in Fascia Iliaca Compartment Block to provide analgesia for positioning femur fracture patients before spinal anesthesia. Al-Azhar International Medical Journal; 2020. https://doi.org/10.21608/aimj.2020.44250.1328.
13. Castillo RL, Ibacache M, Cortínez I, Carrasco-Pozo C, Farías JG, Carrasco RA, et al. Dexmedetomidine Improves Cardiovascular and Ventilatory Outcomes in Critically Ill Patients: Basic and Clinical Approaches. Front Pharmacol. 2020 Feb;10:1641. https://doi.org/10.3389/fphar.2019.01641 PMID:32184718
14. Gupta A, Dwivedi Y, Saxena S, Srivastava U, Mangla S, Mishra S. A randomized control study of dexmedetomidine versus fentanyl as an anesthetic adjuvant in supratentorial craniotomies. Anaesth Pain Intensive Care. 2017;21:306–11.
15. Janatmakan F, Nesioonpour S, Javaherforoosh Zadeh F, Teimouri A, Vaziri M. Comparing the Effect of Clonidine and Dexmedetomidine on Intraoperative Bleeding in Spine Surgery. Anesth Pain Med. 2019 Feb;9(1):e83967. https://doi.org/10.5812/aapm.83967 PMID:30881906
16. Mathew PJ, Jain A, Wig J, Mukherjee KK, Gupta A. Randomized Controlled Trial of Intravenous Dexmedetomidine for Control of Hemodynamics, Surgical Bleeding, and Recovery Profile during Transsphenoidal Pituitary Surgery. J Neuroanaesth Crit Care. 2020;07:091-5.
17. Batra A, Verma R, Bhatia VK, Chandra G, Bhushan S. Dexmedetomidine as an Anesthetic Adjuvant in Intracranial Surgery. Anesth Essays Res. 2017;11(2):309–13. https://doi.org/10.4103/0259-1162.194555 PMID:28663612
18. Tripathi DM, Kumar DV, Malviya D, Singh DM, Kumar DM, Tyagi DA, editors. Hemodynamic and recovery profile with Dexmedetomidine and Fentanyl in intracranial supratentorial surgeries: A comparative stud. 2015.
19. Tanskanen PE, Kyttä JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: a double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006 Nov;97(5):658–65. https://doi.org/10.1093/bja/ael220 PMID:16914460
20. Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth Analg. 2008 Oct;107(4):1340–7. https://doi.org/10.1213/ane.0b013e3181804298 PMID:18806050
21. Jadhav N, Wasekar N, Wagaskar V, Kondwilkar B, Patil R. Use of Dexmedetomidine in Patients Undergoing Craniotomies. J Clin Diagn Res. 2017 Jan;11(1):UC01–08. https://doi.org/10.7860/JCDR/2017/24002.9235 PMID:28274022
22. Chakrabarti D, Kamath S, Madhusudan Reddy KR, Srinivas DB, Manohar N, Masapu D. Effect of adjunctive dexmedetomidine on anesthesia and analgesia requirement and recovery characteristics during Bispectral Index-guided anesthesia for cerebello-pontine angle surgeries: A randomized clinical trial. J Anaesthesiol Clin Pharmacol. 2018;34(4):496–502. https://doi.org/10.4103/joacp.JOACP_55_18 PMID:30774230
23. Amri P, Nahrini S, Hajian-Tilaki K, Hamidian M, Alipour SF, Hamidi SH, et al. Analgesic Effect and Hemodynamic Changes Due to Dexmedetomidine Versus Fentanyl During Elective Colonoscopy: A Double-Blind Randomized Clinical Trial. Anesth Pain Med. 2018 Nov;8(6):e81077. https://doi.org/10.5812/aapm.81077 PMID:30719412
24. J N S, Kumar S, Vijay T. To Compare the Efficacy of Dexmedetomidine Versus Labetalol in Providing Controlled Hypotension in Functional Endoscopic Sinus Surgery. Anesth Pain Med. 2021 Feb;11(1):e108915. https://doi.org/10.5812/aapm.108915 PMID:34221935
25. Sriganesh K, Syeda S, Shanthanna H, Venkataramaiah S, Palaniswamy SR. Comparison of intraoperative fentanyl with dexmedetomidine for perioperative analgesia and opioid consumption during craniotomies: A randomised controlled pilot study with non-inferiority design. Int J Clin Pract. 2019 Jun;73(6):e13338. https://doi.org/10.1111/ijcp.13338 PMID:30829429
26. Sriganesh K, Syeda S, Shanthanna H, Venkataramaiah S, Palaniswamy SR. Effect of Opioid Versus Non-Opioid Analgesia on Surgical Pleth Index and Biomarkers of Surgical Stress During Neurosurgery for Brain Tumors: preliminary Findings. Neurol India. 2020;68(5):1101–5. PMID:33109859
27. Rajan S, Hutcherson MT, Sessler DI, Kurz A, Yang D, Ghobrial M, et al. The Effects of Dexmedetomidine and Remifentanil on Hemodynamic Stability and Analgesic Requirement After Craniotomy: A Randomized Controlled Trial. J Neurosurg Anesthesiol. 2016 Oct;28(4):282–90. https://doi.org/10.1097/ANA.0000000000000221 PMID:26325514
28. Odor PM, Chis Ster I, Wilkinson I, Sage F. Effect of admission fascia iliaca compartment blocks on post-operative abbreviated mental test scores in elderly fractured neck of femur patients: a retrospective cohort study. BMC Anesthesiol. 2017 Jan;17(1):2. https://doi.org/10.1186/s12871-016-0297-8 PMID:28125964
29. Lako SJ, Steegers MA, van Egmond J, Gardeniers J, Staals LM, van Geffen GJ. Incisional continuous fascia iliaca block provides more effective pain relief and fewer side effects than opioids after pelvic osteotomy in children. Anesth Analg. 2009 Dec;109(6):1799–803. https://doi.org/10.1213/ANE.0b013e3181bbc41a PMID:19923505
30. McMeniman TJ, McMeniman PJ, Myers PT, Hayes DA, Cavdarski A, Wong MS, et al.; Brisbane Orthopaedic & Sports Medicine Centre Writing Committee. Femoral nerve block vs fascia iliaca block for total knee arthroplasty postoperative pain control: a prospective, randomized controlled trial. J Arthroplasty. 2010 Dec;25(8):1246–9. https://doi.org/10.1016/j.arth.2009.11.018 PMID:20178889
31. Liu X, Zhang X, Wang X, Wang J, Wang H. Comparative evaluation of intrathecal bupivacaine alone and bupivacaine combined with dexmedetomidine in cesarean section using spinal anesthesia: a meta-analysis. J Int Med Res. 2019 Jul;47(7):2785–99. https://doi.org/10.1177/0300060518797000 PMID:31204535
32. Sivakumar RK, Panneerselvam S, Cherian A, Rudingwa P, Menon J. Perineural vs. intravenous dexmedetomidine as an adjunct to bupivacaine in ultrasound guided fascia iliaca compartment block for femur surgeries: A randomised control trial. Indian J Anaesth. 2018 Nov;62(11):851–7. https://doi.org/10.4103/ija.IJA_397_18 PMID:30532320
33. Murthy TV, Singh R. Alpha 2 adrenoceptor agonist – Dexmedetomidine role in anaesthesia and intensive care: A clinical review. J Anaesthesiol Clin Pharmacol. 2009;25:267–72.
34. Kunisawa T, Ota M, Suzuki A, Takahata O, Iwasaki H. Combination of high-dose dexmedetomidine sedation and fascia iliaca compartment block for hip fracture surgery. J Clin Anesth. 2010 May;22(3):196–200. https://doi.org/10.1016/j.jclinane.2009.02.020 PMID:20400006
35. Andersen JH, Grevstad U, Siegel H, Dahl JB, Mathiesen O, Jæger P. Does Dexmedetomidine Have a Perineural Mechanism of Action When Used as an Adjuvant to Ropivacaine?: A Paired, Blinded, Randomized Trial in Healthy Volunteers. Anesthesiology. 2017 Jan;126(1):66–73. https://doi.org/10.1097/ALN.0000000000001429 PMID:27792047
36. Chinnappa J, Shivanna S, Pujari VS, Anandaswamy TC. Efficacy of dexmedetomidine with ropivacaine in supraclavicular brachial plexus block for upper limb surgeries. J Anaesthesiol Clin Pharmacol. 2017;33(1):81–5. https://doi.org/10.4103/0970-9185.202196 PMID:28413277
37. Sabra M, Abdalla M, Abdelrahman A. Efficacy of ultrasound-guided fascia iliaca compartment block with ropivacaine and dexmedetomidine for postoperative analgesia in hip arthroplasty. Al-Azhar Assiut Med J. 2019;17(4):378–84. https://doi.org/10.4103/AZMJ.AZMJ_101_19.
38. Li Y, Geng J, Wen L, Chen J, Wu Z. Postoperative analgesia with ropivacaine and dexmedetomidine for ultrasound-guided fascia iliaca compartment block after arthroscopic knee surgery. Saudi J Anaesth. 2019;13(2):100–5. PMID:31007654
39. Kumar D, Hooda S, Kiran S, Devi J. Analgesic Efficacy of Ultrasound Guided FICB in Patients with Hip Fracture. J Clin Diagn Res. 2016 Jul;10(7):UC13–6. https://doi.org/10.7860/JCDR/2016/17802.8123 PMID:27630930
40. El-Rahmawy GF, Hayes SM. Efficacy of dexmedetomidine addition to bupivacaine on the quality of blind fascia iliaca compartment block in children undergoing femur fracture surgery. Egypt J Anaesth. 2013;29(2):137–42. https://doi.org/10.1016/j.egja.2012.10.005.

 ORCID
ORCID

