Sepúlveda Haro E.1,*, Bueno García M.S.1, Romero Molina S.1, Ortega Alcántara E.J.1, Guerrero Orriach J.L.1, Cruz Mañas J.1
Recibido: 09-08-2022
Aceptado: 12-10-2022
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 3 pp. 309-314|https://doi.org/10.25237/revchilanestv5213031150
PDF|ePub|RIS
Dexmedetomidina en el implante de válvula aórtica transcatéter. ¿Protege frente a la insuficiencia renal aguda?
Abstract
Introduction: Acute kidney injury (AKI) is a frequent complication after transcatheter aortic valve implantation (TAVI), and it presents a higher risk of myocardial infarction, severe bleeding, transfusión, dialysis, and mortality. Dexmedetomidine has a protective effect on AKI after adult cardiac surgery. We want to study the impact of dexmedetomidine on the incidence of AKI in the postoperative period of TAVI procedure in our center. Methods: We performed a retrospective cohort study comparing the administration of dexmedetomidine (DEX group) versus other sedatives (NO-DEX group) during elective TAVI procedure under transfemoral approach. Results: A total of 122 patients were included in the study. Both groups presented a similar incidence of AKI (19,8% DEX group; 19,2% NO-DEX group; p = 0,949). A subgroup analysis with patients presenting chronic kidney disease showed an AKI incidence of 24%, without statistically significant differences between both groups either. Conclusions: We did not find any difference on AKI incidence, length of hospital stay, 30-day mortality or 12-month mortality in patients undergoing TAVI procedure under sedation with dexmedetomidine compared to other sedatives in our center. It would be interesting to study this hypothetical association through studies with larger samples and better designs.
Resumen
Introducción: La insuficiencia renal aguda (AKI) es una complicación frecuente tras el implante de válvula aórtica transcatéter (TAVI), y presenta un mayor riesgo de infarto agudo de miocardio, sangrado severo, transfusión, diálisis y mortalidad. La dexmedetomidina presenta un efecto protector sobre AKI tras cirugía cardíaca en el adulto. Queremos estudiar el impacto de la dexmedetomidina en la incidencia de AKI en el posoperatorio de procedimiento TAVI en nuestro centro. Métodos: Hemos realizado un estudio de cohorte retrospectiva comparando la administración de dexmedetomidina (grupo DEX) frente a otros sedantes (grupo NO-DEX) durante el procedimiento TAVI electivo con abordaje transfemoral. Resultados: Se incluyeron en el estudio un total de 122 pacientes. Ambos grupos presentaron una incidencia similar de AKI (19,8% el grupo DEX; 19,2% el grupo NO-DEX; p = 0,949). En un análisis de subgrupo en pacientes con insuficiencia renal crónica mostró una incidencia del 24% sin diferencias estadísticamente significativas entre ambos grupos tampoco. Conclusiones: No hemos encontrado ninguna diferencia en la incidencia de AKI, duración de estancia hospitalaria, mortalidad a 30 días y mortalidad a 12 meses en pacientes intervenidos de TAVI bajo sedación con dexmedetomidina comparada con otros sedantes en nuestro centro. Sería interesante estudiar esta hipotética asociación mediante estudios con muestras mayores y mejor diseñados.
-
Introduction
The main indication for transcatheter aortic valve implanta- tion (TAVI) is the severe aortic stenosis in a calcified tricus- pid valve in high-risk surgical patients. It is also a therapeu- tic option for severe aortic insufficiency, severe aortic stenosis in a bicuspid valve, bioprosthetic valve dysfunction, and the clini- cal decision-making should be assessed by the multidisciplinary Heart Team[1].
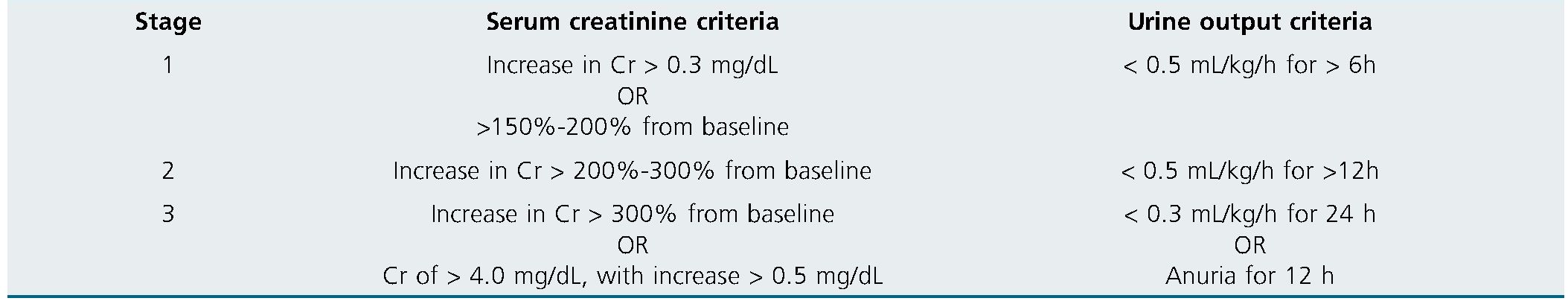
Patients undergoing a TAVI procedure can suffer from several cardiovascular complications in the perioperative pe- riod that require a close surveillance for an early diagnosis and treatment, if required[2],[3]. Acute kidney injury (AKI) is a frequent complication with an estimated incidence of 22,1%, with differences among studies that could be explained by the presence of varied diagnostic criteria, while the Acute Kidney Injury Network (AKIN) and Kidney Disease: Improving Global Outcomes (KDIGO) classification systems are the ones recom- mended by the Valve Academic Research Consortium-2 (VARC- 2)[4],[5]. Patients who suffer from AKI in the postoperative period of TAVI present a higher risk of myocardial infarction, severe bleeding, transfusion, dialysis and mortality[6].
TAVI procedure involves the administration of contrast media, hypotensive episodes during rapid pacing, preoperative volume depletion and catheter manipulation in the aorta of pa- tients with atherosclerosis and risk of embolization. All of these are risk factors associated with AKI[3],[7].
Several other risk factors for AKI after TAVI procedure were identified: transapical approach, chronic kidney disease, class IV of the New York Heart Association (NYHA) classification, pe- ripheral artery disease, atrial fibrillation, congestive cardiac fail- ure, diabetes mellitus, red blood cell transfusion, postoperative thrombocytopenia, systemic inflammatory response syndrome, hypertension, stroke and chronic obstructive pulmonary disease (COPD)[8],[9],[10],[11].
TAVI procedure is usually performed under general an- esthesia or under sedation along with local anesthesia. Pa- tients undergoing sedation with local anesthesia need a lower amount of inotropic agents and a shorter intensive care unit stay than those under general anesthesia, but both techniques show similar complication rates and mortality. For this reason, the current scientific evidence does not support one technique over another[12],[13].
Dexmedetomidine is an alpha-adrenergic agonist with sedative, analgesic and opioid-sparing effects and it is indicat- ed for sedation in critical care units and for procedural seda- tion[14],[15]. Dexmedetomidine has a protective effect on AKI after adult cardiac surgery[16],[17] and it is safe in adults > 65 years[18]. Both propofol and dexmedetomidine are appropriate sedatives for TAVI procedures, as observed in comparative stud- ies[19],[20].
We want to study the impact of dexmedetomidine on the incidence of AKI in the postoperative period of TAVI procedure in our center.
Table 1
-
Methods
We performed a retrospective cohort study comparing the administration of dexmedetomidine (DEX group) versus other sedatives (NO-DEX group) during elective TAVI procedures un- der transfemoral approach between January 2019 and May 2020 in Virgen de la Victoria University Hospital, Spain.
To design an adequate sample recruitment, the period of time selected for the study owes to changes in both the technique of the TAVI procedure and the usual anesthetic management for these interventions in our center. In January 2019 the Cardiology Department begun to use the new-technology recapturable valve prosthesis, and this also conditioned a contrast dose reduction. Also, in the recent years, dexmedetomidine started to be available in our center and, since May 2020, most TAVI procedures are being performed under dexmedetomi- dine-based sedation regimens owing to the anesthesiologists’ preference.
The main outcome was the incidence of AKI in the first 7 postoperative days. Secondary outcomes were the length of postoperative hospital stay, 30-day mortality and 12-month mortality.
Exclusion criteria were: stage 5 chronic kidney disease, ur- gent procedure, acute heart failure, other than transfemoral approach and those in which general anesthesia was the anesthetic plan from the beginning.
Data of the patients included in the study were collected from the digital medical records. We studied several variables: age, sex, weigh, height, obesity according to body mass index, length of hospital stay, chronic kidney disease, baseline creatinine, hypertension, diabetes, NYHA functional class, stroke, atrial fibrillation, left ventricular ejection fraction, American Society of Anesthesiology (ASA) Physical Status classification system, contrast media volume, sedative drugs during the procedure, perioperative complete atrioventricular block, perioperative need for red blood cell (RBC) transfusion, perioperative definitive pacemaker implantation, need for vasoactive drugs, intensive care unit (ICU) admission, extracorporeal kidney-re- placement therapy, the develop of AKI according to VARC-2 criteria (Table 1), 30-day mortality, 12-month mortality and conversion to general anesthesia.
Chronic kidney disease was considered in case it was previously diagnosed or whether a baseline glomerular filtration rate < 60 ml/min/1.73 m2 was detected in the digital medical records. The preoperative baseline creatinine was established as the one upon hospital admission or that after preoperative treatment and recovery of any medical condition present at hospital admission.
The information was processed and analyzed with IBM SPSS Statistics 24. Several descriptive statistics were used: mean, median, mode, quartiles, and percentiles. We also needed different analytical statistics. Comparison between numerical variables showing a normal distribution was performed using the Student’s t-test, while those presenting a non-normal distribution were analyzed with the Mann-Whitney U test after checked with the Kolmogorov-Smirnov test for normality. We used the Chi-squared test to check the independence of two categorical variables, and the Fisher test was used in case the sample size was small. A value of p < 0,05 was considered statistically significant.
-
Results
A total of 122 patients were included in the study (96 be- longing to DEX group and 26 to NO-DEX group). Both groups were similar in terms of age, sex, obesity, hypertension, diabetes mellitus, atrial fibrillation, class IV of the NYHA, stroke, amount of contrast media and conversion to general anesthesia (Table 2).
Preoperative physical status was ASA-IV in the 42.6% of the whole sample, and the rest of them were ASA-III. Only seven patients (5.3%) in the sample had a severely reduced left ventricular ejection fraction (LVEF< 35%), all of whom were included in the DEX group. The incidence of complete atrioventricular block in the sample was 19.7%, and 13.1% of the patients in the sample received a pacemaker implantation in the postoperative period. Vasoactive drugs were needed in 26.2% of the patients in the sample. Admission to the ICU was necessary in 21 (17.2%) patients in the sample.
Prevalence of ASA-IV, severely reduced left ventricular ejection fraction, incidence of complete atrioventricular block, pacemaker implantation, the need for vasoactive drugs, and ICU admission were higher in the DEX group, but these differences were not statistically significant. Perioperative RBC transfusion was administered in 40.2% of the patients, with a non-significant higher incidence in the NO-DEX group.
Dexmedetomidine was always combined with remifentanil, and 41% of patients also received low-dose ketamine. The NO-DEX group was sedated with propofol alone in 58% of cases and different combinations of propofol with midazolam, remifentanil, fentanyl and ketamine were used in the rest of the cases. Only a single patient, who was included in the DEX group, needed conversion to general anesthesia in the context of accidental femoral artery dissection during the procedure. This patient required a vascular repair for which local anesthetic infiltration could not provide enough analgesia. This difference did not reach statistical significance.
Table 2. Patient characteristics
| Dex (n = 96) | No dex (n = 26) | p-value | |
| Preoperative | |||
| Age + | 79.4 ± 7.1 | 80.7 | 0.554 |
| Sex (M:F, n) | 44:52 | 8:18 | 0.168 |
| IMC (kg/m2) + | 28.4 ± 4.8 | 28.6 ± 4.1 | 0.881 |
| Obesity | 27 (35.5%) | 7 (30.4%) | 0.652 |
| Hypertension | 83 (86.5%) | 24 (92.3) | 0.420 |
| Diabetes mellitus | 34 (35.4%) | 10 (38.5%) | 0.774 |
| Atrial fibrillation | 27 (28.1%) | 5 (19.2) | 0.360 |
| NYHA grade IV | 12 (12.5%) | 5 (19,2%) | 0.379 |
| Cerebrovascular disease | 12 (12.5%) | 2 (7.7) | 0.495 |
| FEVI < 35% | 7 (7.3%) | 0 (0%) | 0.344 |
| Chronic kidney disease | 39 (40.6%) | 11 (42.3%) | 0.877 |
| ASA 4 | 45 (46.9%) | 7 (26.9%) | 0.068 |
| Perioperative | |||
| Contrast amount (ml) + | 143.8 ± 52.4 | 152.2 ± 52.1 | 0.491 |
| Auriculoventricular block | 21 (21.9%) | 3 (11.5%) | 0.240 |
| Definitive pacemaker need | 14 (14.6%) | 2 (7.7) | 0.518 |
| Conversion to general anesth | 1 (1%) | 0 (0%) | 1 |
| Red blood cells transfusion | 37 (38.5%) | 12 (46.2%) | 0.482 |
| Admission at ICU | 19 (19.8%) | 2 (7.7%) | 0.240 |
| Vasopressor drugs usage | 29 (30.2%) | 3 (11.5%) | 0.055 |
Data is expressed as n (percentage)/ +: mean ±SD / Statistical significance established as p < 0.05.
Only 24 patients (19.7%) suffered from AKI in the postoperative period (Table 3).
Except for one patient who presented AKI-II (NO-DEX group), the rest of the patients had the lowest degree of kidney dysfunction, AKI-I. None of the patients required extracorporeal kidney-replacement therapy in the postoperative period. Both groups presented a similar incidence of AKI (19.8% DEX group; 19.2% NO-DEX group; p = 0.949). A subgroup analysis with patients presenting chronic kidney disease showed an AKI incidence of 24%, without statistically significant differences be- tween both groups (25.6% DEX group; 18.2% NO-DEX group; p = 1).
The postoperative hospital length of stay in our sample had a median of three days, corresponding to 47.5% of the patients. The minimum length of stay was two days, and the maximum was 20 days. In 75% of the patients in the sample the length of stay was five days or less. Median hospital length of stay in the NO-DEX group was lower than the DEX group, with a non-significant statistical difference.
Only two patients (each of them belonging to a different group) died in the first 30 postoperative days, corresponding to a mortality of 1.6% in the sample, without differences between the two groups. A patient in the DEX group died in the immediate postoperative period in the context of acute pulmonary oedema. The other patient, belonging to the NO-DEX group, died on the sixth postoperative day in the ward in the context of AKI-II. In the first postoperative year, a sum of 15 patients (12.3%) died, without differences between the two groups.
Table 3
| Outcomes | Dex (n = 96) | No dex (n = 26) | p-value |
| Acute kidney injury | 19 (19.8%) | 5 (19.2%) | 0.949 |
| Length of hospital stay p | 4 [4-6] | 3 [3-4.5] | 0.233 |
| 30-day mortality | 1 (1%) | 1 (3.8%) | 0.382 |
| 12-month mortality | 12 (12.5%) | 3 (11.5%) | 1 |
Data is expressed as n (percentage) / p: median [interquartile range] / Statistical significance established as p < 0.05.
-
Discussion
A meta-analysis of randomized controlled trials concluded that perioperative administration of adult patients undergo- ing cardiac surgery may reduce the incidence of postoperative AKI[16]. Recently, another randomized controlled trial regarding the impact of dexmedetomidine on AKI in the postoperative of adult aortic surgery showed a protective effect of this sedative on kidney function along with a reduction on length of hospital stay, without any significant increase of side effects[17]. Contrary to what we expected, in our study we have not observed any difference on AKI incidence when using dexmedetomidine in the perioperative of TAVI under sedation. Moreover, we have not identified any differences on length of hospital stay, 30-day mortality or 12-month mortality. We could not find any paper studying the influence of dexmedetomidine on perioperative kidney function in TAVI procedures, so our investigation would probably be the first one on this topic.
In our study, the DEX group had a higher prevalence of ASA-IV physical status and severely reduced left ventricular ejection fraction, and although these differences did not reach the statistical significance, it seems that the anesthesiologists were prone to choose a dexmedetomidine-based sedation for patients with higher comorbidity.
Incidence of AKI in our study was 19.7%, which is slightly lower than the incidence of 22.1% reported in a recent meta- analysis on AKI in patients undergoing TAVI procedures. Of note, except for a patient who suffered from AKI-II, the rest of the patients presented the least severity of kidney dysfunction, corresponding to an AKI-I. While the meta-analysis showed a 5.8% incidence of extracorporeal kidney-replacement therapy, in our sample none of the patients needed it[6].
Recently, the marketing authorisation holders for dexmedetomidine-containing products in agreement with the Euro- pean Medicines Agency informed an increased risk of mortality in ventilated critically ill adult ICU patients under sedation with dexmedetomidine in the age group < 65 years compared with alternative sedatives[22]. This warning was based on SPICE III, a randomized controlled trial which also reported a 90-day mortality reduction in ventilated critically ill ICU adult patients under sedation with dexmedetomidine in the age group > 65 years compared with alternative sedatives[18]. In our study, 95% of patients were > 65 years and 12-month mortality was 12.5%, without any difference between the groups, and this involves a lower rate of that found (17.8%) in other studies about TAVI procedures[23].
We did not find any difference between the groups regarding the incidence of conversion to general anesthesia, and the only patient who needed it was owing to a surgical complication.
It would be interesting to study an hypothetical influence of dexmedetomidine on the development of complete atrio- ventricular block during TAVI procedure, as we found a higher incidence in the DEX group with a non-significant statistical difference. It is possible that an additive effect between the mechanical compressive effect of the implanted bioprosthetic aortic valve and the pharmacological depressant effect of dexmedetomidine on the atrioventricular node could increase the risk of complete atrioventricular block. As far as we know, this has not been studied before.
Our study presents some limitations. The sample size is reduced, specially the NO-DEX group, as the recruitment is limited by the technical change in TAVI procedures and the anesthesiologists’ sedation regimen preferences, as detailed in the
methods section. This could involve a low statistical potency to detect any possible association between dexmedetomidine and kidney function and also the secondary outcomes: length of hospital stay, 30-day mortality and 12-month mortality.
Owing to the continuous surveillance needed during TAVI procedures, the recording of vasoactive drugs in the anesthesia record sheets is sometimes incomplete, which could influence the data collection. Also, the lack of systematic measurement of the urine output in the ward limited the diagnostic criteria of AKI, so serum creatinine was the only criteria used. Finally, this is a retrospective study and, as such, it presents the intrinsic limitations of this kind of study.
-
Conclusions
We did not find any difference on AKI incidence, length of hospital stay, 30-day mortality or 12-month mortality in patients undergoing transfemoral TAVI procedure under sedation with dexmedetomidine compared to other sedatives in our cen- ter. It would be interesting to study this hypothetical association through studies with larger samples and better designs.
References
1. Sundt TM, Jneid H. Guideline Update on Indications for Transcatheter Aortic Valve Implantation Based on the 2020 American College of Cardiology/American Heart Association Guidelines for Management of Valvular Heart Disease. JAMA Cardiol. 2021 Sep;6(9):1088–9. https://doi.org/10.1001/jamacardio.2021.2534 PMID:34287627
2. Ussia GP, Scarabelli M, Mulè M, Barbanti M, Cammalleri V, Immè S, et al. Postprocedural management of patients after transcatheter aortic valve implantation procedure with self-expanding bioprosthesis. Catheter Cardiovasc Interv. 2010 Nov;76(5):757–66. https://doi.org/10.1002/ccd.22602 PMID:20506545
3. Raiten JM, Gutsche JT, Horak J, Augoustides JG. Critical care management of patients following transcatheter aortic valve replacement. F1000 Res. 2013;2:62. https://doi.org/10.12688/f1000research.2-62.v1.
4. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al.; Valve Academic Research Consortium-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013 Jan;145(1):6–23. https://doi.org/10.1016/j.jtcvs.2012.09.002 PMID:23084102
5. Koifman E, Segev A, Fefer P, Barbash I, Sabbag A, Medvedovsky D, et al. Comparison of acute kidney injury classifications in patients undergoing transcatheter aortic valve implantation: predictors and long-term outcomes. Catheter Cardiovasc Interv. 2016 Feb;87(3):523–31. https://doi.org/10.1002/ccd.26138 PMID:26268940
6. Gargiulo G, Sannino A, Capodanno D, Perrino C, Capranzano P, Barbanti M, et al. Impact of postoperative acute kidney injury on clinical outcomes after transcatheter aortic valve implantation: A meta-analysis of 5,971 patients. Catheter Cardiovasc Interv. 2015 Sep;86(3):518–27. https://doi.org/10.1002/ccd.25867 PMID:25641565
7. Rahman MS, Sharma R, Brecker SJ. Transcatheter aortic valve implantation in patients with pre-existing chronic kidney disease. Int J Cardiol Heart Vasc. 2015 Apr;8:9–18. https://doi.org/10.1016/j.ijcha.2015.04.006 PMID:28785672
8. Aregger F, Wenaweser P, Hellige GJ, Kadner A, Carrel T, Windecker S, et al. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant. 2009 Jul;24(7):2175–9. https://doi.org/10.1093/ndt/gfp036 PMID:19211648
9. Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochellière R, Doyle D, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010 Apr;31(7):865–74. https://doi.org/10.1093/eurheartj/ehp552 PMID:20037180
10. Scherner M, Wahlers T. Acute kidney injury after transcatheter aortic valve implantation. J Thorac Dis. 2015 Sep;7(9):1527–35. https://doi.org/10.3978/j.issn.2072-1439.2015.06.14 PMID:26543598
11. Wang J, Yu W, Zhou Y, Yang Y, Li C, Liu N, et al. Independent Risk Factors Contributing to Acute Kidney Injury According to Updated Valve Academic Research Consortium-2 Criteria After Transcatheter Aortic Valve Implantation: A Meta-analysis and Meta-regression of 13 Studies. J Cardiothorac Vasc Anesth. 2017 Jun;31(3):816–26. https://doi.org/10.1053/j.jvca.2016.12.021 PMID:28385646
12. Hyman MC, Vemulapalli S, Szeto WY, Stebbins A, Patel PA, Matsouaka RA, et al. Conscious Sedation Versus General Anesthesia for Transcatheter Aortic Valve Replacement: Insights from the National Cardiovascular Data Registry Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2017 Nov;136(22):2132–40. https://doi.org/10.1161/CIRCULATIONAHA.116.026656 PMID:28864443
13. Erkan G, Ozyaprak B, Kaya FA, Dursun İ, Korkmaz L. Comparison of anesthesia management in transcatheter aortic valve implantation: a retrospective cohort study. Braz J Anesthesiol. 2021: S0104-0014(21)00273-6. https://doi.org/10.1016/j.bjane.2021.06.017..
14. Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs. 2011 Jul;71(11):1481–501. https://doi.org/10.2165/11207190-000000000-00000 PMID:21812509
15. Keating GM. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs. 2015 Jul;75(10):1119–30. https://doi.org/10.1007/s40265-015-0419-5 PMID:26063213
16. Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 2018 Jan;18(1):7. https://doi.org/10.1186/s12871-018-0472-1 PMID:29334927
17. Soh S, Shim JK, Song JW, Bae JC, Kwak YL. Effect of dexmedetomidine on acute kidney injury after aortic surgery: a single-centre, placebo-controlled, randomised controlled trial. Br J Anaesth. 2020: S0007-0912(20)30001-5. https://doi.org/10.1016/j.bja.2019.12.036..
18. Shehabi Y, Serpa Neto A, Howe BD, Bellomo R, Arabi YM, Bailey M, et al.; SPICE III Study Investigators. Early sedation with dexmedetomidine in ventilated critically ill patients and heterogeneity of treatment effect in the SPICE III randomised controlled trial. Intensive Care Med. 2021 Apr;47(4):455–66. https://doi.org/10.1007/s00134-021-06356-8 PMID:33686482
19. Khalil M, Al-Agaty A, Asaad O, Mahmoud M, Omar AS, Abdelrazik A, et al. A comparative study between propofol and dexmedetomidine as sedative agents during performing transcatheter aortic valve implantation. J Clin Anesth. 2016 Aug;32:242–7. https://doi.org/10.1016/j.jclinane.2016.03.014 PMID:27290982
20. Mayr NP, Wiesner G, van der Starre P, Hapfelmeier A, Goppel G, Kasel AM, et al. Dexmedetomidine versus propofol-opioid for sedation in transcatheter aortic valve implantation patients: a retrospective analysis of periprocedural gas exchange and hemodynamic support. Can J Anaesth. 2018; 65(6):647-657. English. https://doi.org/10.1007/s12630-018-1092-4..
21. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al.; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. https://doi.org/10.1186/cc5713 PMID:17331245
22. European Medicines Agency (EMA). Dexmedetomidine: Increased risk of mortality in intensive care unit (ICU) patients ≤65 years [Internet]. Amsterdam, The Netherlands. 16/06/2022 [consulted 24/07/2022]. Available at: https://www.ema.europa.eu/en/medicines/dhpc/dexmedetomidine-increased-risk-mortality-intensive-care-unit-icu-patients-65-years
23. Kleczyński P, Bagieński M, Dziewierz A, Rzeszutko Ł, Sorysz D, Trębacz J, et al. Twelve-month quality of life improvement and all-cause mortality in elderly patients undergoing transcatheter aortic valve replacement. Int J Artif Organs. 2016 Oct;39(8):444–9. https://doi.org/10.5301/ijao.5000521 PMID:27716868

 ORCID
ORCID