Edith Labos1,a, Romina Albite1,b, Angel Golimstok1,c, Alejandro Renato1,d, M. Cecilia Fernández1, Francisco Bonofiglio1,*
Recibido: 25-05-2023
Aceptado: 02-07-2023
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 5 pp. 497-504|https://doi.org/10.25237/revchilanestv52n5-08
PDF|ePub|RIS
El acto anestésico quirúrgico aumenta el deterioro cognitivo en pacientes ancianos comparados con población sana
Abstract
Background and Objective: The purpose of this study was to investigate whether anesthetic surgical act is a risk factor that contributes to the development of postoperative cognitive dysfunction (POCD) in elderly patients, or whether there are pre-existing conditions that predispose it. Materials and Methods: The study involved a non-randomized prospective cohort of patients over 65 years of age undergoing surgery/anes- thesia (as well as a group of healthy individuals who were not exposed to either anesthesia or surgery). The study was carried out at the Hospital Italiano Buenos Aires between April 2017 and March 2020, and the data was collected in the anesthesia service. A total of 200 participants were evaluated, 100 in each group. A battery of cognitive tests was administered at baseline and 12 months later. Cognitive impairment was assessed with scales and analyzed by multivariate and principal component analysis. Results: At 12 months, exposed patients had a 36% incidence of deterioration, while unexposed patients had only 8%. The odds ratio was 6.5 (95% CI 2.9-15.8 p < 0.001). Adjusting for increased to 8.6 (95% CI 3.6-22.9 p < 0.001). Main components were identified that were correlated with the cognitive scales. The crude relative risk derived from the odds ratio was 4.5, while the adjusted relative risk was 7.3. Conclusions: The findings validate the existence of durable cognitive performance results in patients who were exposed. It is essential to stress the importance of performing a cognitive assessment before administering anesthesia and/or surgery to identify vulnerable people and continue monitoring their evolution.
Resumen
Introducción y Objetivo: El propósito de este estudio fue investigar si el acto quirúrgico anestésico es un factor de riesgo que contribuye al desarrollo de disfunción cognitiva posoperatoria (DCPO) en pacientes ancianos, o si existen condiciones preexistentes que la predisponen. Materiales y Métodos: El estudio involucró una cohorte prospectiva no aleatorizada de pacientes mayores de 65 años sometidos a cirugía/anestesia (así como un grupo de individuos sanos que no estuvieron expuestos ni a la anestesia ni a la cirugía). El estudio se realizó en el Hospital Italiano Buenos Aires entre abril de 2017 y marzo de 2020, y los datos fueron recolectados en el servicio de anestesia. Se evaluaron un total de 200 participantes, 100 en cada grupo. Se administró una batería de pruebas cognitivas al inicio y 12 meses después. El deterioro cognitivo se evaluó con escalas y se analizó mediante análisis multivariante y de componentes principales. Resultados: A los 12 meses, los pacientes expuestos tenían una incidencia de deterioro del 36%, mientras que los pacientes no expuestos tenían solo 8%. El odds ratio fue de 6,5 (IC 95% 2,9-15,8 p < 0,001). El ajuste aumentó a 8,6 (IC del 95 %: 3,6-22,9 p < 0,001). Se identificaron componentes principales que se correlacionaron con las escalas cognitivas. El riesgo relativo bruto derivado del odds ratio fue de 4,5, mientras que el riesgo relativo ajustado fue de 7,3. Conclusiones: Los hallazgos validan la existencia de resultados duraderos de rendimiento cognitivo en pacientes que estuvieron expuestos. Es fundamental recalcar la importancia de realizar una evaluación cognitiva antes de administrar anestesia y/o cirugía para identificar a las personas vulnerables y seguir monitoreando su evolución.
-
Introduction
It is generally accepted that memory slowly decreases with age. There are more than 100 known causes that can deepen or accelerate this condition, among which can be described: stress, surgery/anesthesia, diabetes, depression, hypothyroidism, medications, arrhythmias, sleep apnea, etc.[1]. The vast majority of patients with memory disorders are related to Alzheimer’s disease[2] ,vascular disorders( dementia)[3] among other conditions.
It has adopted the new nomenclature, which has been published regarding postoperative cognitive dysfunction[4]. This work recommends that “perioperative neurocognitive disorders” be used as an overarching term for cognitive impairment identified in the preoperative (before operation) or postoperative period (up to 12 months).
To the best of our knowledge, the first report linking cognitive dysfunction to general anesthesia (GA) and surgery dates from 1955.
In recent decades, 2 relevant international studies have been published examining this association: International Study of Postoperative Cognitive Dysfunction 1 and 2 (ISPOCD 1 and 2). ISPOCD 1 seems to confirm what was previously reported, showing that, out of the total number of patients over 65 years of age studied, a percentage of 26% presented POCD in the first week and close to 10% still continued at the third month[5]. Meanwhile in the ISPOCD 2, it was concluded that in the first week the percentage of patients older than 65 years under GA with cognitive alterations amounted to 20% while those who received regional anesthesia only reached 10%[6].
Despite the importance of this topic for decision-making, the literature is still not conclusive in demonstrating whether POCD is directly related to techniques such as spinal, epidural, regional, and combined plus neuraxial[7], as well as whether it is related to general anesthesia using inhalation agents[8], or with the depth effect of anesthesia[9], with the reduction of other sedative and anesthetic drugs, with the reduction of opioids[10], with cerebral perfusion and cerebral oxygen saturation[11] or if it is related to cerebral perfusion and cerebral saturation of oxygen 11 nor is it clear whether there are preconditions that facilitate its presence after global l[12].
The objective of this research was to study the anesthetic surgical act that facilitates the development of postoperative cognitive dysfunction in the elderly, and to identify previous conditions that could influence the appearance of POCD.
-
Methods
A non-randomized prospective cohort study of participants older than 65 years in the Italian Hospital Medical Care Program (IHMCP) was conducted. The population selected for this study completed at least 6 years of formal education.
The study, carried out in the period from April 2017 to March 2020, was approved by the local Research Protocol Ethics Committee on June 23, 2016 (No. 2813) and registered with Clinicaltrials.gov (Identifier NCT03067259) on March 2, 2017. Informed consent was obtained from all participants. The investigation has been conducted following the principles of the Declaration of Helsinki.
The entire study population was interviewed at the time of recruitment, recording their medical and medication history.
The participants were divided into 2 groups: exposed and not exposed to an anesthetic surgical act. Participants were assessed at 2 time points: 1) after study entry before surgery (T1) and 2) 12 months after surgery (T12) to determine the incidence of POCD in each group. The exposed group was defined as those patients with elective outpatient surgery, or elective surgery hospitalized or in closed units. This group of patients was selected from the list of our hospital surgery and anesthetic procedures program. The unexposed group (healthy control group) was recruited from among those patients in the program who had not had a surgery/anesthesia program in the last 12 months. This group of participants was randomly selected from a database of non-surgical specialties.
The exclusion criteria were verified from the patient’s medical records. The excluded patients were those who did not give their consent; those with a Montreal Cognitive Assessment (MOCA)[13] < 21; those with a previous diagnosis of dementia, psychosis or depression symptoms checked with the Geriatric Depression Scale (GDS)[14] patients with a history of substance abuse; marked decrease in visual or hearing acuity; patients who were receiving antipsychotics or who have changed the dose of any psychoactive drug in the last 30 days and those patients with oncological disease and life expectancy less than 1 year.
The patients were personally evaluated in the office, between 7 days and 24 hours before surgery and later, they were evaluated with the same methods at 12 months. The scales that were used consisted of a standardized test instruments measuring attentional, executive, and verbal memory functions: MOCA, Memory Impairment Screen (MIS)[15], Trail Making Test (TMT)[16], GDS, Activities of daily living (ADL)[17], Instrumental Activities of Daily Living (IADL)[18], Assessment of frailty[19] (Annex 1).
Patients were evaluated by trained neuropsychologists. Primary outcome measures: Cognitive change was considered when the patient presented alterations in at least one of the cognitive and functional battery tests at 12 months.
-
Statistical analysis
This research uses and adheres to the reporting guideline, STROBE[20]. To determine the incidence of patients with cognitive impairment one year after surgery, an alpha of 0.05 and a power of 80% were used, estimating a sample size of 100 in each group. For the calculation of the sample, the software STATA version 13 was extracted.
In the descriptive analysis, the quantitative data were expressed as mean and standard deviation (SD) or as median and interquartile range 25-75 (IQR) according to their distribution. Qualitative data were expressed in absolute and relative frequency in percentage. To assess the association between exposure to surgery under general anesthesia and cognitive decline, logistic regression analysis was performed. Odds ratios were expressed with their 95% confidence interval (95% CI). To identify the variables involved in changes in cognitive tests and their degree of statistical significance, adjustment variables were age, sex, years of education, high blood pressure, baseline cognitive tests, and functional scales: deferred MIS, TMT, Clock drawing test, MOCA, Fluency (phonological verbal fluency), ADL, IADL, and the presence of Frailty. The odds ratio can be used to calculate the relative risk, and a Principal Component Analysis (PCA) was conducted on both exposed and unexposed groups to explore variables before conducting regression analysis to confirm hypotheses. Categorical variables were transformed into numerical ones, with a cut-off value of 0.50
used for the analysis. In the subgroup of patients with cognitive decline, correlations between changes in cognitive scales were measured using Pearson’s coefficient. The statistical analysis was performed using R software version 4.0.3.
-
Results
A total of 290 subjects underwent evaluation in this study, with 100 patients allocated to both the exposed group and the unexposed group as illustrated in the STROBE participant flow diagram. The characteristics of the participants are detailed in Table 1.
Clinical results. In the study group, the patients underwent various types of surgeries, with the highest percentage being abdominal surgeries (31%), followed by genitourinary surgeries (29%), thoracic surgeries (19%), head and neck surgeries (7 %), endoscopies (6%), cardiology procedures (4%), trauma surgeries (3%) and neurology surgeries (1%).
The average duration of the surgeries was 87.5 minutes, ranging from a minimum of 10 minutes to a maximum of 430 minutes.
The average duration of anesthesia was 120 minutes, with a range from a minimum of 24 minutes to a maximum of 460 minutes.
Cognitive results. The prevalence of cognitive impairment was significantly higher in the exposed group (36%) compared to the unexposed group (8%) with a p value less than 0.001. The odds ratio was 6.5 (95% CI 2.9-15.8, p < 0.001), and after adjusting for age, sex, years of education, hypertension, and baseline tests, the odds ratio increased to 8.6 (95% CI: 3.622.9, p < 0.001). The relative risk calculated from the odds ratio was 4.5 in the crude analysis and 7.3 in the adjusted analysis.
At 12 months, neurocognitive tests were administered to participants in both groups.
Table 1 Characteristics of the participants exposed and unexposed group with p
|
Characteristics |
Unexposed n = 100 |
Exposed n = 100 |
P |
|
Age (mean sd) |
74.89 (6.3) |
74.21 (5.45) |
0.415 |
|
Years of education (mean sd) |
12.7 (2.5) |
13 (2,4) |
0.387 |
|
Sex; female n (%) |
58 |
46 |
0.119 |
|
Comorbidities (%) |
|||
|
Arterial hypertension |
5 |
67 |
< 0.001 |
|
Diabetes |
3 |
12 |
0.032 |
|
Dyslipidemia |
14 |
50 |
< 0.001 |
|
Heart disease |
21 |
50 |
< 0.001 |
|
Previous chemotherapy |
37 |
7 |
< 0.001 |
|
Previous surgery |
11 |
16 |
0.408 |
|
Medication comsuption (%) |
|||
|
Corticoids |
17 |
0 |
< 0.001 |
|
Antidepressants |
7 |
7 |
1 |
|
Benzodiazepines |
25 |
27 |
0.872 |
|
Opiates |
2 |
0 |
0.477 |
|
sd: standard deviation; n: number. |
|||
In the unexposed group, 8 participants showed cognitive changes, 4 with normal baseline scores and 4 with lower scores. The tests that presented the greatest decrease in this group were TMT, MIS, fluency, and frailty.
In the exposed group, 36 participants showed cognitive changes at 12 months. Of these, 24 had baseline scores within normal limits, while the other 12 had worsening low baseline scores. Two of these 12 patients progressed to dementia. The tests that showed the most changes in this group were fluency, MOCA, and the clock drawing test (see MOCA test).
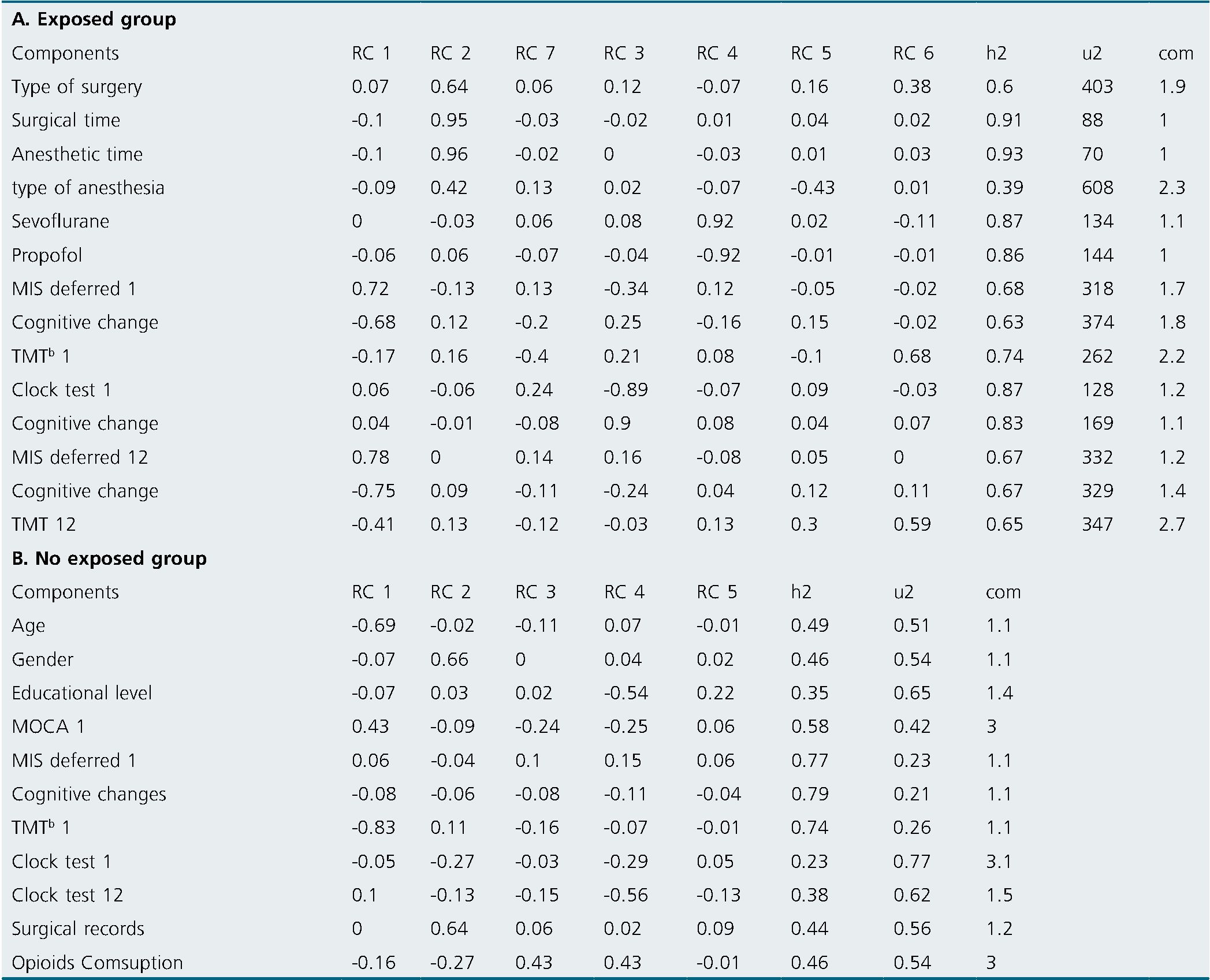
Principal component results. Exposed group: In the principal component analysis, 4 components were identified that were correlated with the cognitive scales, while 1 was related to the surgical/anesthetic procedure. These are represented in Figure 1, and Table 2 presents the comprehensive principal component analysis. Principal component analysis revealed several correlations between cognitive performance and certain factors. Component 1 (A) showed a negative correlation between baseline deferred MIS at time 1 and cognitive change at 12 months, whereas immediate memory phase (initial MIS 12) was positively correlated with deferred memory phase (Deferred MIS 12). Component 3(B) demonstrated a direct correlation between baseline TMT at time 1 and cognitive change, while baseline delayed MIS at time 1 was inversely correlated with cognitive change. Component 4 (C) showed a correlation between baseline deferred MIS at time 1, baseline clock test at time 1, and cognitive change. A low score on the clock drawing test and on the MIS were correlated with greater cognitive change. Component 6(D) showed that patients with high scores on baseline delayed MIS at time 1 and who received less anesthesia had higher TMT at 12 months and higher delayed MIS at 12 months. Component 7 (E) was associated with the surgical procedure and showed that the greater the severity of the surgery, the worse TMT at baseline and at 12 months. Non-exposed group: In the principal component analysis, 1 component correlated with the cognitive scales. This correlated opioid use and educational level with the clock test. The less time in formal education and the greater the consumption of opioids, the less efficiency in the clock test at 12 months (Figure 2)
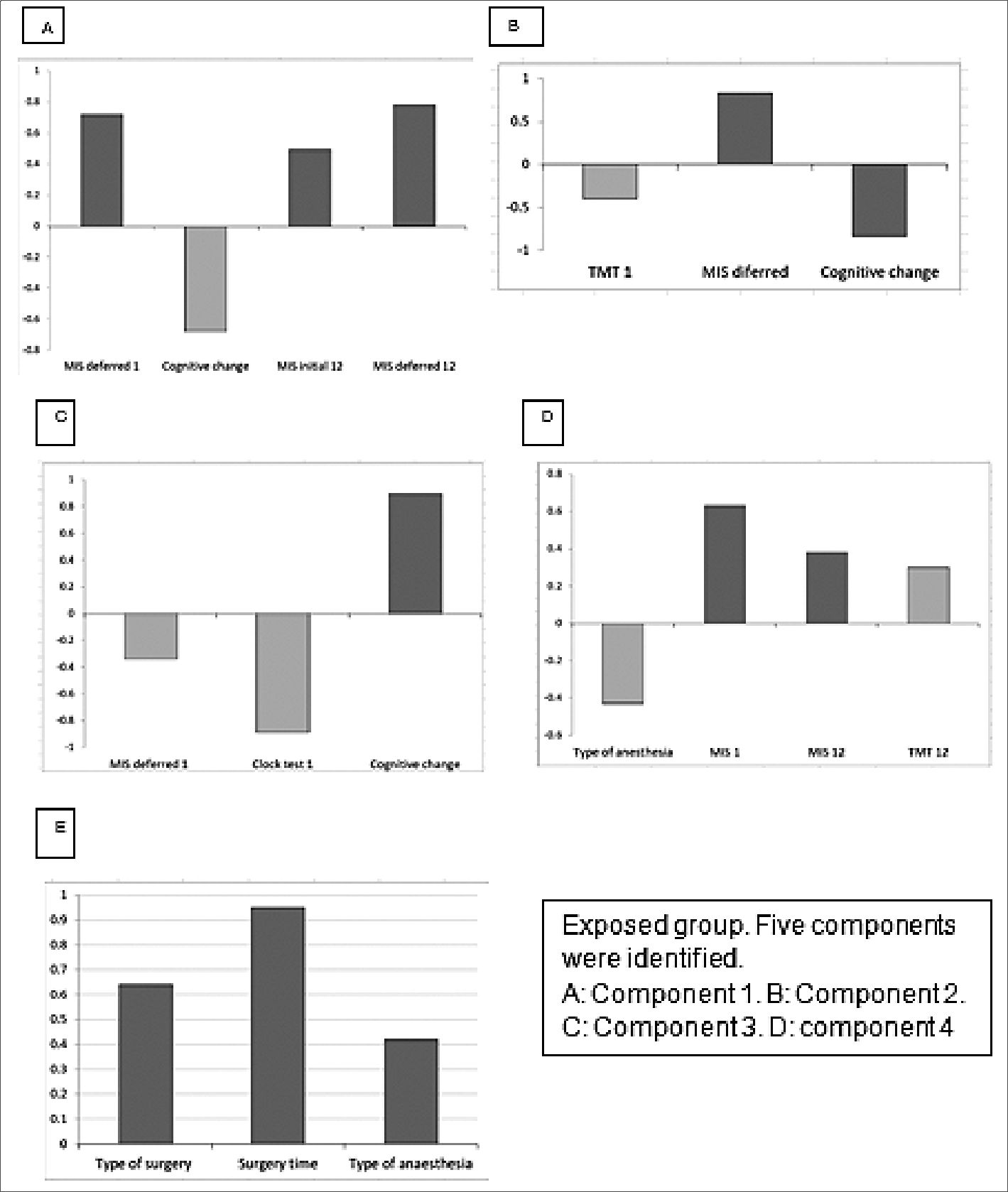
Figure 1. Principal component analysis.
-
Discussion
The clinical evidence of our study shows that 36% of the patients exposed to anesthetic surgical act suffered a decline in their cognitive performance at 12 months, configuring a mild degree of POCD and 2% of the patients a serious degree of POCD.
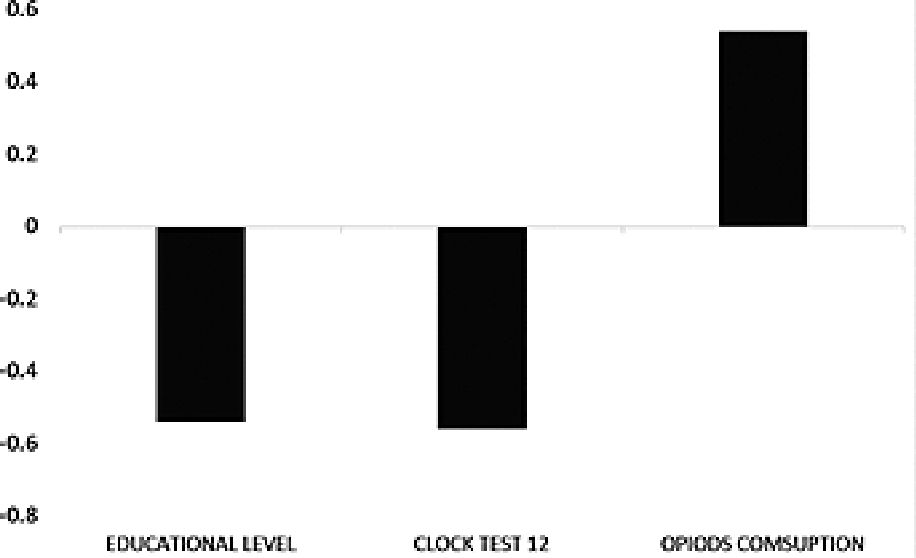
Figure 2. Principal analysis components. No exposed group. 1 component were identified.
Table 2. Principal component analysis
The battery of tests used to assess POCD in the literature is highly variable and there is no defined consensus on the tests to be used. In 2020, the Society for Perioperative Assessment and Quality Improvement (SPAQI) convened experts and identified a handful of appropriate and accepted cognitive assessment tools to identify cognitive impairment[21]. In this research, the same cognitive assessment tools proposed by SPAQI were used.
According to previous publications, the alteration of cognitive function occurs more frequently from ages over 65 years[22],[23]. In this population, it has been described that in addition to the age, duration, and complexity of the surgery , the drugs and the anesthetic technique used[7],[8],[24] are also related to postoperative cognitive changes[25].
It is accepted that the onset of the POCD is around the 7th day. In the elderly population, it occurs with an incidence of around 30% in the first week, decreasing to 1% per year (long-acting POCD) in non-cardiac patients[26]. In our study, the percentage of POCD per year amounted to 36%, and we did not evaluate the patients at shorter times since it was not the objective of this study. The differences between both investigations may be due to the fact that our population was older, 75, while in the other it was about 68.
It has been suggested that to prevent the appearance of POCD, intraoperative care should be improved, with actions such as: minimizing anesthetic depth and limiting its duration[27]. However, it is not clear if these measures are sufficient to prevent the appearance of POCD or if greater intraoperative attention is required to prevent this disease.
The conclusions of the investigations are controversial: while there are articles that could not associate cognitive deterioration after major non-cardiac surgery with the surgical procedure or the anesthetic technique[28], others instead associate cognitive changes in patients older with the type of anesthesia used[29].
It was reported in previous papers that people with previous cognitive impairment had a higher risk of developing POCD 3 months after the procedure. With reference to this, in this study, 25% of the participants in the exposure group who met the inclusion criteria had decreased baseline scores on one or more domain scales. This group presented a greater decline at 12 months. These results are within the values reported for non-cardiac surgeries 30 and below the percentage of cardiac surgeries[30]. The same fact was registered in 4 participants (4%) of the control group who presented a low score in one or more baseline tests and which decreased even more at 12 months.
The initial data analysis affirms that having low cognitive performance initially can result in the development of POCD later on. The data from both groups of patients suggest that both populations experience cognitive decline solely due to aging. However, the percentage of patients who develop POCD after 12 months is higher in the exposed group compared to the control group (36% vs 8%). Changes in cognitive function were observed to a greater extent in tasks related to executive function.
The basic functional scales and instrumental scales, which measure the ability to perform routine tasks, did not indicate any evidence of functional impairment. One consideration is that, according to previous studies[18], these scales may lack sufficient sensitivity to detect subtle functional changes.
In the principal component analysis of the exposed group, component 1 (A) stands out, indicating that a better cognitive state of the patient at the beginning would serve as a protective factor against later dysfunctions. Component 3 (B) supports these findings by suggesting that good performance on benchmark tests is associated with the absence of cognitive deficits at 12 months. Component 4 (C) demonstrates that a low score in executive function (assessed by the clock test) and memory (MIS) at baseline is associated with greater cognitive deficits at 12 months. Component 6 (D) correlates with the type and duration of anesthesia exposure, revealing that less exposure is associated with better global cognitive performance. Lastly, the final component 7 (E) reveals that the complexity of the surgery and the degree of exposure to anesthesia are related to worse performance at 12 months.
In the principal component analysis of the non-exposed group (healthy group), there are no significant correlations either at the beginning or at 12 months, since they obey all but one explainable cause. This is the consumption of opioids and the educational level with cognition tests, this correlation reinforces the working hypothesis.
The relationship between patient sex and surgical history (component 3) may be attributed to the uneven distribution of men and women who underwent surgery in the sample.
The principal component analysis performed on both groups tells us that no association was found between the results of the control group and the exposed group.
Coinciding with other investigations[23],[31], our results were associated with antihypertensive and hypoglycemic medication, a history of hypertension and heart disease, age, and the presence of previous cognitive dysfunction.
In the clinical theoretical framework, a possible hypothesis in the correlation of the previous cognitive state, with the profile of long-term progression, is the concept of Cognitive Reserve (CR)[32]. It can be inferred, then, that the subjects with the best level of CR could better compensate for their cognitive performance, in the face of the risk of anesthetic exposure. The idea that this reserve would act as a protector becomes relevant when observing the clinical response of exposed patients and its variability in relation to pre-existing conditions.
The study revealed interesting findings related to the characterization of the POCD cognitive profile and the identification of scales and tests that can predict long-term deficits. Specifically, the MIS Test, which assesses immediate and delayed memory, the TMT, which evaluates attention, and the MOCA, a scale for evaluating global cognitive performance, were identified as good predictors of POCD.
Many more questions remain to be answered, so this topic is not concluded. More specific research is required to answer them.
The research has several strengths, including the prospective evaluation of participants for POCD and confirmation of findings through clinical evaluations, which minimizes the risk of misclassification. Another strength is that it was evaluated 12 months after surgery, providing valuable long-term insights into the condition. As there are few long-term studies on POCD, the results of this study are crucial for understanding the condition.
Some weaknesses of our study that should be taken into account. First, we cannot separate the effects of anesthesia/ surgery from the cognitive ones. Another is that the included patients were only exposed to general anesthesia, there is no comparison with any other type of anesthesia. This is a project for the future, since if we found a similar effect with other anesthetic agents, we could implicate surgical exposure as the main culprit for cognitive change in patients. Another weakness is that we have not carried out evaluations in intermediate terms during the 12 months and this prevents us from drawing conclusions about the evolution of the impairment. On the other hand, we lack brain imaging tests and biomarkers to better characterize impairment.
In conclusion, in this longitudinal follow-up of a population of older adults, under general anesthesia, mild POCD was associated in the long term with age, antihypertensive and hypoglycemic medication, history of hypertension and heart disease, type of surgery, anesthesia time, and previous cognitive dysfunction.
Future research should aim to identify intraoperative situations that lead to POCD, emphasizing the comorbidities of the patients.
Acknowledgements: The present work was performed in simultaneity with the Medical Clinic, Neurology and Anesthesiology service of the Hospital Italiano de Buenos Aires. The following professionals participated in the design and execution: Daniel Seinhart, Gustavo García Fornari, Marcelo Schapira, Vanina Pagotto, Marina Cavagna, Carla Sesarini, Maximiliano Smietniansky, Marcela Fernández, Maria Elvira Soderlund, Waleska Berrios, Yasmin Tenaglia, Yanina Esquef, Enriqueta Chouhy Oria, Rubén Vallejos, Lourdes Posadas Martínez, Diego Giunta, Antonella Bonofiglio, Lucas Sánchez, Irene Blanc and Lucila Laudani.
This study was approved by the local Research Protocol Ethics Committee (CEPI) (No. 2813; approval date: 23/06/2016) and registered at clinicaltrials.gov (Identifier NCT03067259; approval date: 02/03/2017).
Funding: This work was supported by BECAS SALUD INVESTIGA Abraam Sonis/2017
[RENIS registration code: ISOO1527].
Conflict of interest: The authors have no conflicts of interest.
-
References
1. Morley JE. An Overview of Cognitive Impairment. Clin Geriatr Med. 2018 Nov;34(4):505–13. https://doi.org/10.1016/j.cger.2018.06.003 PMID:30336985
2. Jahn H. Memory loss in Alzheimer’s disease. Dialogues Clin Neurosci. 2013 Dec;15(4):445–54. https://doi.org/10.31887/DCNS.2013.15.4/hjahn PMID:24459411
3. Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, et al. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017 Jan;177(1):51–8. https://doi.org/10.1001/jamainternmed.2016.6807 PMID:27893041
4. Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, et al.; Nomenclature Consensus Working Group. Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery-2018. Anesthesiology. 2018 Nov;129(5):872–9. https://doi.org/10.1097/ALN.0000000000002334 PMID:30325806
5. Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al.; International Study of Post-Operative Cognitive Dysfunction. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. Lancet. 1998 Mar;351(9106):857–61. https://doi.org/10.1016/S0140-6736(97)07382-0 PMID:9525362
6. Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, et al.; ISPOCD2(International Study of Postoperative Cognitive Dysfunction) Investigators. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003 Mar;47(3):260–6. https://doi.org/10.1034/j.1399-6576.2003.00057.x PMID:12648190
7. Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimers Dis. 2010;22(s3 Suppl 3):67–79. https://doi.org/10.3233/JAD-2010-101086 PMID:20858956
8. Miller D, Lewis SR, Pritchard MW, Schofield-Robinson OJ, Shelton CL, Alderson P, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev. 2018 Aug;8(8):CD012317. https://doi.org/10.1002/14651858.CD012317.pub2 PMID:30129968
9. Lu X, Jin X, Yang S, Xia Y. The correlation of the depth of anesthesia and postoperative cognitive impairment: A meta-analysis based on randomized controlled trials. J Clin Anesth. 2018 Mar;45:55–9. https://doi.org/10.1016/j.jclinane.2017.12.002 PMID:29275267
10. Ma D, Rajakumaraswamy N, Maze M. alpha2-Adrenoceptor agonists: shedding light on neuroprotection? Br Med Bull. 2005 Jan;71(1):77–92. https://doi.org/10.1093/bmb/ldh036 PMID:15684247
11. Chernov VI, Efimova NY, Efimova IY, Akhmedov SD, Lishmanov YB. Short-term and long-term cognitive function and cerebral perfusion in off-pump and on-pump coronary artery bypass patients. Eur J Cardiothorac Surg. 2006 Jan;29(1):74–81. https://doi.org/10.1016/j.ejcts.2005.10.001 PMID:16337804
12. Belrose JC, Noppens RR. Anesthesiology and cognitive impairment: a narrative review of current clinical literature. BMC Anesthesiol. 2019 Dec;19(1):241. https://doi.org/10.1186/s12871-019-0903-7 PMID:31881996
13. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695–9. https://doi.org/10.1111/j.1532-5415.2005.53221.x PMID:15817019
14. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37–49. https://doi.org/10.1016/0022-3956(82)90033-4 PMID:7183759
15. Edith L, Sofía T, Daniel S, Marcelo S, Alejandro R. Spanish Version of Test MIS (*) with Delayed Memory Recall Normative Values and Results in a Population with Mild Cognitive Impairment [Internet]. Vol. 04. Adv Alzheimer Dis. 2015;4(2):45–61. https://doi.org/10.4236/aad.2015.42006.
16. Smith SR, Servesco AM, Edwards JW, Rahban R, Barazani S, Nowinski LA, et al. Exploring the validity of the comprehensive trail making test. Clin Neuropsychol. 2008 May;22(3):507–18. https://doi.org/10.1080/13854040701399269 PMID:17853128
17. Edemekong PF, Bomgaars DL, Sukumaran S, Schoo C. Activities of Daily Living. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
18. Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983 Dec;31(12):721–7. https://doi.org/10.1111/j.1532-5415.1983.tb03391.x PMID:6418786
19. Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006 Sep;35(5):526–9. https://doi.org/10.1093/ageing/afl041 PMID:16757522
20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008 Apr;61(4):344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008 PMID:18313558
21. Arias F, Wiggins M, Urman RD, Armstrong R, Pfeifer K, Bader AM, et al. Rapid In-Person Cognitive Screening in the Preoperative Setting: Test Considerations and Recommendations from the Society for Perioperative Assessment and Quality Improvement (SPAQI) [Internet]. Perioper Care Oper Room Manag. 2020 Jun;19:100089. https://doi.org/10.1016/j.pcorm.2020.100089 PMID:32342018
22. Cottrell JE, Hartung J. Anesthesia and Cognitive Outcome in Elderly Patients: A Narrative Viewpoint. J Neurosurg Anesthesiol. 2020 Jan;32(1):9–17. https://doi.org/10.1097/ANA.0000000000000640 PMID:31490337
23. Murman DL. The Impact of Age on Cognition. Semin Hear. 2015 Aug;36(3):111–21. https://doi.org/10.1055/s-0035-1555115 PMID:27516712
24. Whitlock EL, Grisell Diaz-Ramirez L, Avidan MS. Surgery and persistent cognitive decline: a commentary and an independent discussion. Br J Anaesth. 2020 Mar;124(3):229–34. https://doi.org/10.1016/j.bja.2019.10.020 PMID:31839254
25. Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008 Jan;108(1):18–30. https://doi.org/10.1097/01.anes.0000296071.19434.1e PMID:18156878
26. Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 2011 Aug;17(4):376–81. https://doi.org/10.1097/MCC.0b013e328348bece PMID:21716111
27. Brodier EA, Cibelli M. Postoperative cognitive dysfunction in clinical practice. BJA Educ. 2021 Feb;21(2):75–82. https://doi.org/10.1016/j.bjae.2020.10.004 PMID:33889433
28. Konishi Y, Evered LA, Scott DA, Silbert BS. Postoperative cognitive dysfunction after sevoflurane or propofol general anaesthesia in combination with spinal anaesthesia for hip arthroplasty. Anaesth Intensive Care. 2018 Nov;46(6):596–600. https://doi.org/10.1177/0310057X1804600610 PMID:30447669
29. Hovens IB, Schoemaker RG, van der Zee EA, Heineman E, Izaks GJ, van Leeuwen BL. Thinking through postoperative cognitive dysfunction: how to bridge the gap between clinical and pre-clinical perspectives. Brain Behav Immun. 2012 Oct;26(7):1169–79. https://doi.org/10.1016/j.bbi.2012.06.004 PMID:22728316
30. Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS; ISPOCD Group. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009 Mar;110(3):548–55. https://doi.org/10.1097/ALN.0b013e318195b569 PMID:19225398
31. Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al.; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011 Sep;42(9):2672–713. https://doi.org/10.1161/STR.0b013e3182299496 PMID:21778438
32. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002 Mar;8(3):448–60. https://doi.org/10.1017/S1355617702813248 PMID:11939702

 ORCID
ORCID