Tarek Abdelsalam Seleem MD.1, Mohamed Abdelgawad Abdelhalim Aboelsuod MD.1, Mohamed Hussienny Mahmoud MD.2, Mostafa Shaaban Abdelazeem1*
Recibido: —
Aceptado: —
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 6 pp. 592-598|https://doi.org/10.25237/revchilanestv52n6-06
PDF|ePub|RIS
Abstract
Background: Cardioplegia is crucial for protecting the heart. Long-term cardiac protection is offered by the Del Nido Cardioplegia solution (dNCS). Aim: To compare conventional crystalloid cardioplegia with modified del Nido cardioplegia in mitral valve regurgitation replacement surgery. Objectives and Methods: This randomized clinical study included 80 patients undergoing to elective mitral regurgitation replacement surgery of both sex and age from 21 to 60 years with ASA (III and IV). All 80 patients were randomly assigned to receive conventional cardioplegia (St. Thomas’ cardioplegic solutions) or to obtain a modified del Nido cardioplegia (using normal saline) (groups A and B, respectively). The laboratory data for assessment of myocardial protection were obtained through measurements of preoperative serum levels of cardiac enzymes as, CK-MB, troponin, and lactate, immediately after operation, 12 and 24 hours postoperatively. Results: There was a significant increase in CK-MB only after 1 h (p < .001) in group (A) and after 12 h. CK-MB and lactate levels were significantly increased (p < 0.05). Also, after 24 h. CK-MB and Troponin T levels were significantly increased (p < .001). Regarding complications, one patient had atrial fibrillation and one case had permanent stroke in group (A), and two patients died in group (A), while one patient died in group (B) without a significant difference between the groups. Conclusions: We report that Modified Del Nido Cardioplegia has better postoperative chemical parameters as CK-MB, Troponin T and Lactate in mitral valve surgery in adults. DNC is a safe alternative to conventional cardioplegia and has achieved at least the same results.
Resumen
Introducción: La cardioplejía es crucial para proteger el corazón. La solución Del Nido Cardioplegia (dNCS) podría ofrecer mejor protección cardíaca a largo plazo. Objetivo: Comparar la cardioplejía cristaloide convencional con la cardioplejía del Nido modificada en la cirugía de recambio valvular mitral. Objetivos y Métodos: Este estudio clínico aleatorizado incluyó a 80 pacientes sometidos a cirugía de recambio valvular mitral electiva de ambos sexos y edades de 21 a 60 años con ASA (III y IV). Los 80 pacientes fueron asignados al azar para recibir cardioplejía convencional (soluciones cardiopléjicas de St. Thomas) o cardioplejía del Nido modificada (usando solución salina normal) (grupos A y B, respectivamente). Los datos de laboratorio para la evaluación de la protección miocárdica se obtuvieron a través de la medición de los niveles séricos preoperatorios de enzimas cardíacas como CK-MB, troponina y lactato, inmediatamente después de la operación y 12 y 24 h después de la operación. Resultados: Hubo un aumento significativo de CK-MB solo después de 1 h (p < 0,001) en el grupo (A) y después de 12 h. Los niveles de CK-MB y lactato aumentaron significativamente (p < 0,05). Además, pasadas las 24 h. Los niveles de CK-MB y troponina T aumentaron significativamente (p < 0,001). En cuanto a las complicaciones, un paciente presentó fibrilación auricular y un caso ictus permanente en el grupo (A), y dos pacientes fallecieron en el grupo (A), mientras que un paciente falleció en el grupo (B) sin diferencia significativa entre los grupos. Conclusiones: Reportamos que la Cardioplejía Del Nido Modificada tiene mejores parámetros químicos postoperatorios como CK-MB, Troponina T y Lactato en cirugía de válvula mitral en adultos. DNC es una alternativa segura a la cardioplejía convencional y ha logrado al menos los mismos resultados.
-
Introduction
Cardioplegia is essential for successful surgical outcomes because it protects the heart, slows metabolic activity, and increases the resistance of the myocardium to ischemia over extended periods of time[2].
The timing of cardioplegia administration is highly important to prevent myocardial dysfunction. A conventional multidose cardioplegia should be given every 15 to 20 min. Time is essential in open-heart surgery, and any disruption to the flow of the procedure, even if only for a few seconds, before each cardioplegia supply wastes precious minutes[3].
Finding a treatment that would permit longer re-dosing intervals, a longer time during which the heart could be safely ischemic, and similar myocardial protection would therefore be the best solution[4].
The inhibition of fast-acting sodium channels or calcium- activated processes is the mechanism by which crystalloid cardioplegic solutions induce cardiopulmonary arrest. Use for hyperkalemia in the St. Thomas Hospital solution and variants, blocking fast-acting sodium channels[5].
Crystalloid cardioplegia, such as St. Thomas’ cardioplegic solution No. 2 (traditional cardioplegia), has been widely used by cardiac surgeons; nevertheless, it must be injected multiple times at frequent intervals. It has been observed that the postoperative prognosis is negatively affected by increases in myocardial acidity between doses. Therefore, it would be preferable if the time between cardioplegic doses could be lengthened, thereby decreasing the total number of doses administered[6].
Since the early 1990s, juvenile cardiac doctors have made effective use of del Nido cardioplegia solution (dNCS) due to its ability to provide long-term cardiac protection with a single dose [7]. The goal of our research was to match the effectiveness of modified del Nido cardioplegia to traditional crystalloid cardioplegia during mitral valve replacement surgery.
-
Material & Methods
In this potential Randomized, clinical trial research, include 80 cases scheduled for elective mitral regurgitation replacement surgery with normal coronaries of both sex and age from 21 to 60 years with ASA III and IV classification, were selected from attendee of cardiothoracic surgery clinics of Al-Azhar Hospitals during August 2022 to April 2023.
The total number was randomized by simple randomization into two groups: group (A) had 40 cases who received conventional cardioplegia (St. Thomas’ cardioplegic solutions) and group (B) contained 40 patients who received normal salinebased modified del Nido cardioplegia.
-
Inclusion criteria
– Age: 21 – 50 years.
– Patients scheduled for elective mitral regurgitation replacement with normal coronaries.
-
Exclusion criteria
– Patients with renal disease.
– Left ventricular ejection fraction less than 50%.
– Previous cardiac surgery.
– Patient with BMI > 30.
– Severe psychiatric diseases.
– Inability to give informed consent for participation.
-
Methods
All patients had been subjected to complete history taking and complete physical examination. Each patient had been received the previously prepared cardioplegia according to his group.
Saline based del Nido formulation had been prepared as Nakao et al.[8], formula, and St. Thomas’ formulation had been prepared as Mishra et al.[9] formula. Depending on the individual reason, the cardioplegic solutions were given anterogradely at the coronary ostia or root of the aorta. Just prior to their administration, the St. Thomas’ Cardioplegia and Normal Saline Based Modified del Nido were altered at the time of service.
Typically, the solutions had been delivered at a temperature of 4° C, with a system pressure of 100-200 mmHg & an taking flow of 200-300 mL/min, with an extreme dose of 1,000 mL for cases weighing over 50 kg. Further dosages were administered every 20 minutes for St. Thomas (traditional cardioplegia), and after 90 minutes for modified del Nido if necessary.
-
Outcomes study:
Primary outcome: Assess myocardial protection through measurements of the serum levels of cardiac enzymes as CK- MB, troponin, and Lactate preoperative, just after the operation, 12 and 24 hours post-operative.
Secondary outcomes: Assessments of additional myocardial protection measures were done using ICON (noninvasive hemodynamics electrical cardiometry) which are Ejection Fraction (EF), Stroke volume (SV), the incidence of Stroke volume variation (SVV), Cardiac output (COP) and Cardiac index (CI). All every 2 hrs in first 6 hrs then every 6 hrs in first 24 h postoperative.
-
Sample size
This study base on the research carried out by Mishra et al.[9] Epi-Info STATCALC was used to sample size calculated by considering the following assumptions: – 95% two-sided confidence level, with a power of 80% & a error of 5% odds ratio calculated = 1,115. The final maximum sample size taken from the Epi-Info output was 72. Thus, the sample size was increased to 80 subjects to assume any drop out cases during follow up (Figure 1).
-
Ethical approval
Each patient had given their informed consent and the study had been approved by Institutional Review Board (IRB) (No.00255/2022) with accepted at clinical trial.org (NCT05797090).
-
Statistical analysis:
The information was entered into Statistical Program for the Social Sciences for codification, processing, and analysis (SPSS2) 1st edition, IBM, United States. Descriptive statistics were calculated. We compare between both groups by Chi-square test for categorical variables, Student T-test for normally distributed quantitative variables, Mann Whitney test for abnormally distributed quantitative variables and friedman’s test (Fr test) for continuous data to test for significant difference between more than two dependent non- parametric data along different time points.
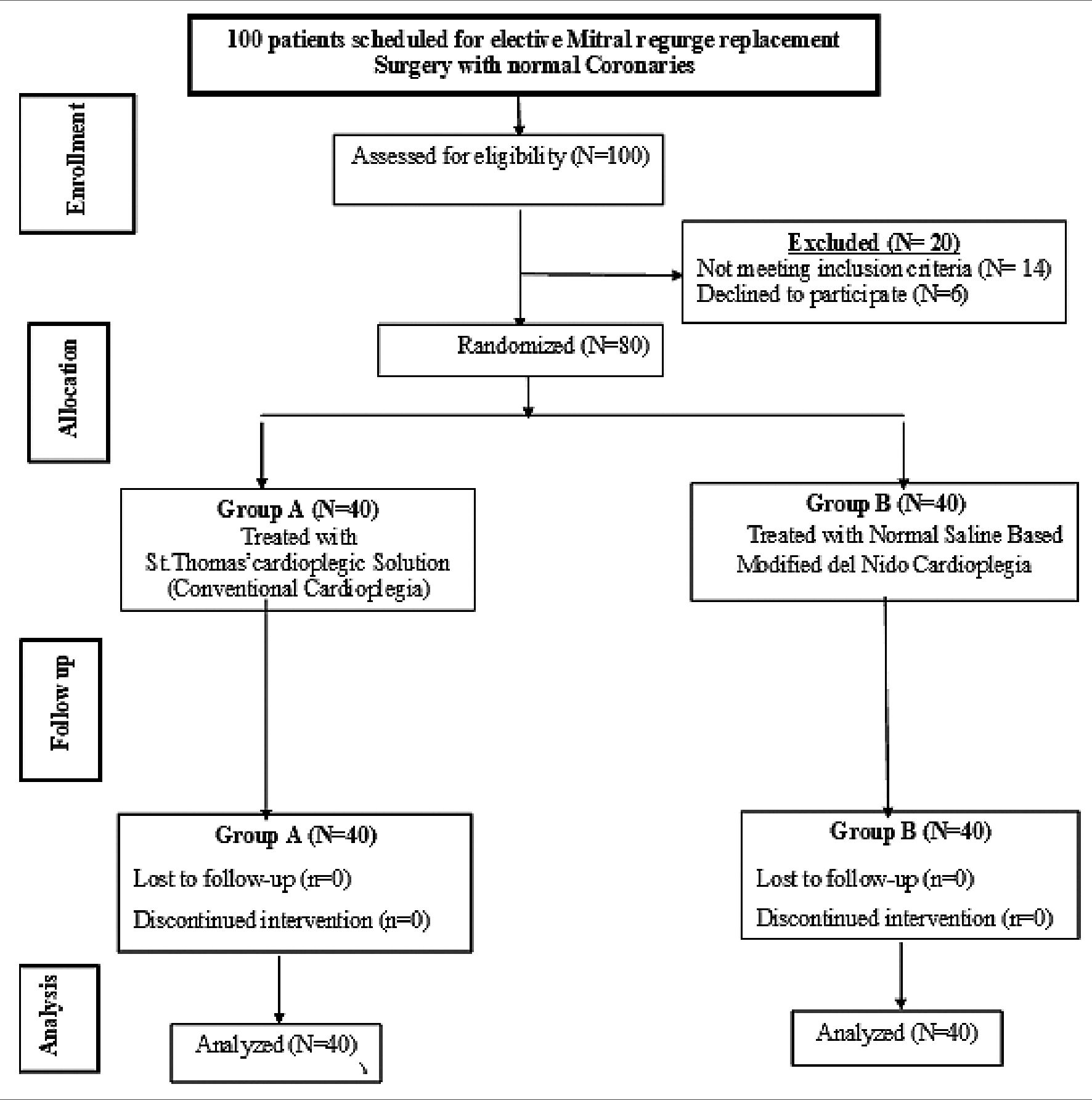
Figure 1. Study flow chart.
-
Results
Eighty participants were split evenly between two groups for this investigation. There were 55% male & 45% women in group (A), with a median age of 49.75 ± 7.11 years; in group (B), the median age was 48.72 ± 8.51 years. Age, sex, ASA classification, body mass index, functional class according to the New York Heart Association, mitral regurgitation grade, cardiovascular comorbidity, and non-cardiac comorbidities did not differ significantly among the two groups (Table 1).
There was no significant difference between groups (A) and (B) in ejection fraction measured preoperatively and after 2 h postoperative. Meanwhile, there was a significant increase in ejection fraction in group (B) matched to group (A) after 4, 6, 12, 18 and 24 h postoperative (p = 0.017, 0.001, < 0.001, < 0.001 and < 0.001, respectively).
Preoperative, 2, 4, and 6-hour measures of stroke volume found no significant differences among groups (A) & (B). Meanwhile there was significant increase of stroke volume in group (A) matched to group (B) after 12, 18 and 24 h postoperative (p = 0.023, < 0.001 and < 0.001, respectively). Also, no significant difference was showed among group (A) & group (B) regarding cardiac output measured preoperative, after 2, 4, 6, 12, 18 and 24 h postoperative.
Postoperative inotropic support was used in 50% cases in group (A) & 40% cases in group (B). The mean number of inotropes was 1.43 ± 0.6 in group A & 1.39 ± 0.5 in group B. No significant difference was showed among the 2 groups according to postoperative inotropic support or number of inotropes (Table 2).
Table 1. Comparison of demographic and clinical data between groups
| Variables | Group (A) (n = 40) | Group (B) (n = 40) | P-value | ||
| n | % | n | % | ||
| Age (year) Mean± SD | 49.75± 7.11 | 48.72± 8.51 | |||
| Median | 50.0 | 49.5 | 0.388 | ||
| Range | 30.0 – 61 | .0 | 32.0 – 67.0 | ||
| Sex Male | 22 | 55.0% | 24 | 60.0% | 0.651 |
| Female | 18 | 45.0% | 16 | 40.0% | |
| ASA classification III | 28 | 70% | 24 | 60% | 0.428 |
| IV | 12 | 30% | 16 | 40% | |
| BMI (Kg/m2) Mean ± SD | 26.93± 1.65 | 26.98± 1.79 | |||
| Median | 27.0 | 27.0 | 0.815 | ||
| Range | 24.0 – 31.0 | 24.0 – 30.0 | |||
| NYHA functional I | 18 | 45.0% | 21 | 52.5% | |
| class ii | 12 | 30.0% | 9 | 22.5% | |
| 0.657 | |||||
| III | 8 | 20.0% | 6 | 15.0% | |
| IV | 2 | 5.0% | 4 | 10.0% | |
| Mitral regurgitation Grade I | 2 | 5.0% | 1 | 2.5% | |
| rade Grade II | 11 | 27.5% | 10 | 25.0% | |
| Grade III | 9 | 22.5% | 7 | 17.5% | 0.487 |
| Grade IV | 16 | 40.0% | 15 | 37.5% | |
| with MS | 2 | 5.0% | 7 | 17.5% | |
| Cardiovascular No | 21 | 52.5% | 26 | 65.0% | |
| comorbidity af or flutter | 1 | 2.5% | 0 | 0.0% | |
| 0.551 | |||||
| HTN | 16 | 40.0% | 12 | 30.0% | |
| PAD | 2 | 5.0% | 2 | 5.0% | |
| Non-cardiac No | 29 | 72.5% | 29 | 72.5% | 1.00 |
| comorbidity dm | 11 | 27.5% | 11 | 27.5% | |
BMI: Body mass index; ASA: American Society of Anesthesiologists; NYHA: New York Heart Association; AF: Atrial fibrillation; HTN: Hypertension, PAD: Peripheral Arterial Disease; DM: Diabetes mellitus.
Table 2. Comparison of postoperative inotropic support between the studied groups
| Variable | Group (A) (n = 40) | n | Group (B) (n = 40) | % | P-value | |||
| n | % | |||||||
| Postoperative inotropic support | No | 20 | 50.0% | 24 | 60.0% | 0.369 | ||
| Yes | 20 | 50.0% | 16 | 40.0% | ||||
| Number of inotropes | Mean ± SD | 1.43 ± 0.60 | 1.39 ± 0.50 | 0.723 | ||||
| Median | 1.0 | 1.0 | ||||||
| Range | 0.0- 2.0 | 1.0- 2.0 | ||||||
One unit of total PRBC Transfusion was needed for 40% case in group (A) & 15% cases in group (B) while two units was needed for 7.5% cases in group (A) only. Total PRBC Transfusion was significantly higher in group A matched to group B (Table 3).
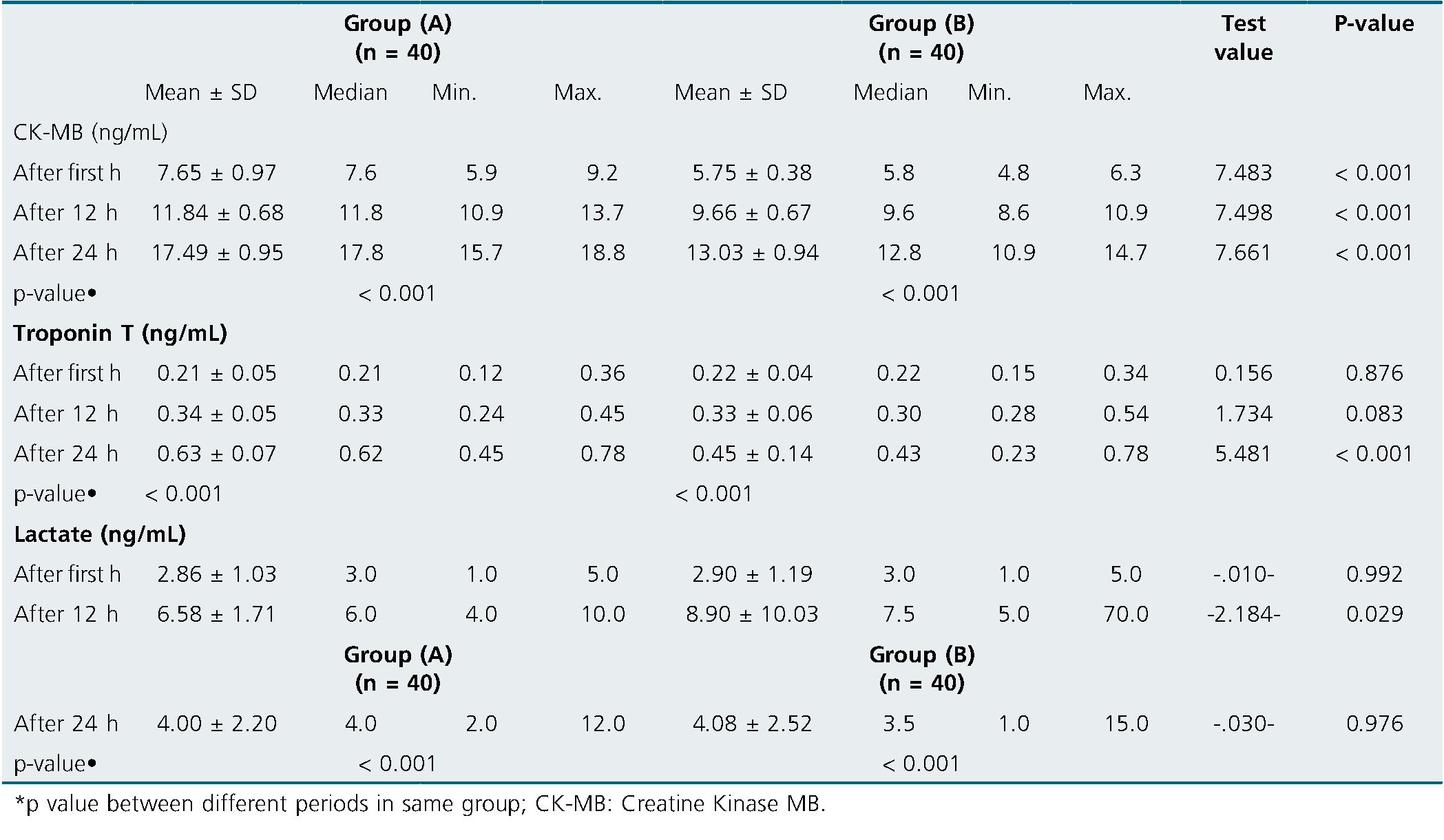
There was significant increase of CK-MB in group (A) compared to group (B) after 1, 12 and 24 h postoperative (p < 0.001 for all). There was significant increase of Troponin T in group (A) matched to group (B) after 24 h only (p < 0.001). Also, there was significant rise of Lactate in group (A) compared to group (B) after 12 h only (p = 0.029) (Table 4).
Table 3. Comparison of postoperative Total PRBC Transfusion between groups
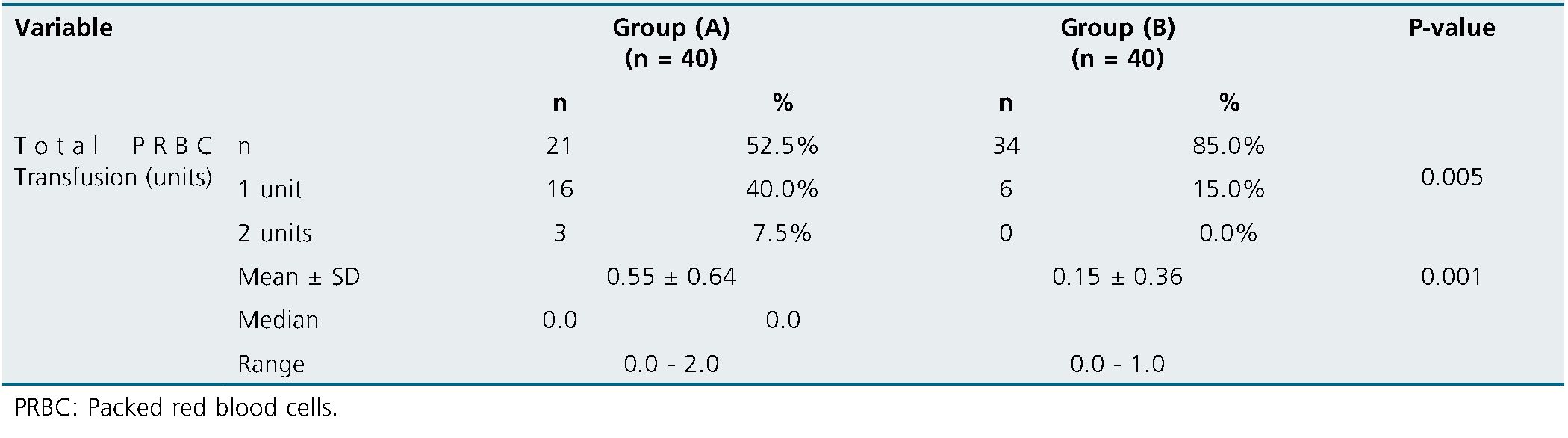
Table 4. Comparison between the studied groups regarding CK-MB, Troponin T and Lactate at different follow-up periods
The mean ventilation duration was 6.92 ± 0.62 h in group A & 6.60± 0.68 h in group B. The ICU stay was 1.21 ± 0.35 days in group A & 1.14 ± 0.36 days in group B. The mean postoperative hospital stays were 3.70 ± 0.72 days in group A & 3.83 ± 0.98 days in group B. By matching group (A) with group (B), we found no statistically significant differences in terms of ventilation time, intensive care unit time, or overall hospital time.
One patient in group (A) experienced atrial fibrillation, and another in group (B) experienced a permanent stroke (B). The mortality rate in group (A) was 5% while in group (B) was 2.5%. When comparing complications in groups A & B, there was no statistically significant difference.
-
Discussion
Heart surgery requires cardioplegia to stop the heart and create a sterile operating environment free of blood. Traditional blood cardioplegia consists of potassium-rich solutions that induce cardiac arrest while cells stay in a depolarizer condition, preventing the propagation of myocardial electrical activity. Myocardial protection from the solution requires dosage every 20-30 minutes[10]. In this work, we aimed to compare between conventional crystalloid cardioplegia with modified del Nido cardioplegia in mitral valve regurgitation replacement surgery.
According to our findings, no statistically significant difference was showed among the 2 groups according to potassium, glucose, creatinine and Cockcroft-Gault creatinine clearance (p > 0.05). Hemoglobin and hematocrit were significantly decrease in group A matched to group B (p < 0.001). No significant difference was showed among the 2 groups according to preoperative TIA or stroke (p > 0.05) as preoperative TIA or stroke was seen in 15% cases in group (A) & in five% in group (B). This came in line with[11].
Comparable with our findings, Haranal et al.[12], investigated One hundred patients who qualified were split into two groups: those who received del Nido cardioplegia and those who received BSTH cardioplegia solution. and stated that there was no significant difference as regard age, sex, BMI & cardiac complications (stroke).
Also, Floh et al.[13], observed that the primary cardiac diagnosis was insignificantly different between both studied groups.
Regarding our findings, no significant difference was found among group (A) & group (B) concerning ejection fraction measured preoperative and after 2 h (p > 0.05). Meanwhile there was significant rise of ejection fraction in group (B) matched to group (A) after 4 h (p = 0.017), after 6 h after 12 h, after 18 h, & after 24 h. In each group, there was significant differences in ejection fraction between preoperative measurements and postoperative follow up measurements.
This came in line with Haranal et al.[12], who found preoperative EF was 66 + 8.1 in Del Nido group and was 66 + 9.2 in in BSTH with no significant difference between both groups however, Discharge 2D-echocardiography showed no significant difference in left ventricular ejection fraction (LVEF) among the del Nido group (65.6% + 8.9%) and the BSTH group (65.7% + 8.2%). Population diversity may account for this difference.
Our research presented that no significant difference was showed among the 2 groups according to postoperative inotropic support or number of inotropes (p > 0.05). This was in line with[11],[12].
In the present study, total PRBC Transfusion was significantly higher in group A matched to group B. This came in line with[12].
In the present research, there was significant rise of CK-MB in group (A) compared to group (B) after 1 h after 12 h and after 24 h (p < 0.001). In each group, there was significant steadily rise in CK-MB levels after 12 & 24 h compared to its level at first h. No significant difference was found among group (A) and group (B) regarding Troponin T measured after 1 h, and after 12 h (p > 0.05). Meanwhile there was significant rise of Troponin T in group (A) matched to group (B) after 24 h (p < 0,001). In each group, there was significant steadily increase in Troponin T levels after 12 & 24 h compared to its level at first h.
Troponin secretion As a diagnostic biomarker for myocardial survival, I is widely employed. Del Nido cardioplegia solution use was associated with decreased troponin levels in the juvenile population, according to a study by O’Brien and his associates[14].
In the study by Haranal et al.[12], Troponin T levels were somewhat greater in the BSTH cardioplegia group, However, there was no distinction among the groups in terms of the timing of troponin T release. Two solutions of del Nido and a blood-based Saint Thomas presented no difference in troponin levels when compared in a randomized controlled experiment[15].
Del Nido patients had lower rates of ventricular fibrillation after aortic cross-clamp removals, lower CK-MB values, lower glucose levels during CPB or less need for postoperative intravenous insulin, 34, lower need for trans operative inotropic support, and lesser troponin levels compared to patients treated with conventional blood cardioplegia[16].
In the same line with our finding, Kavala and Turkyilmaz’s Kavala and Turkyilmaz[11], demonstrated that the DNC group’s CK-MB levels at 1 & 24 h postoperatively were significantly lesser than those of the BC group (DNC = 5.82 ± 4.72 ng/mL and BC = .27± 4.69 ng/mL, respectively, P = 0.041; DNC = 13.30 ± 7.72 ng/mL and BC= 17.53 ± 7.26 ng/mL During one h, there was no difference in the troponin levels among the groups (DNC = 0.13 ± 0.05ng/mL and BC = 0.24 ± 0.25 ng/mL, P = e0.099). Troponin levels in the DNC group were considerably lower after 24 h postoperatively (DNC = 0.28 ± 0.41ng/mL and BC = 0.67 ± 1.03ng/mL, P = .001).
In the present research, there was no discernible difference among group (A) and group (B) concerning lactate measurements taken after one hour and after twenty-four h (p > 0.05). After 12 h, there was a noticeable drop in lactate in group (A) matched to group (B). (p = 0.029). Each group’s lactate levels significantly increased over the course of 12 and 24 h compared to their initial levels.
Unfortunately, the Plasma Lyte used as the basic solution for del Nido cardioplegia is not available in all countries, preventing its use in many cardiac facilities. An observational study by Kantathut et al.[18], compared the del Nido cardioplegia technique with the use of Ringer lactate as the base solution for myocardial preservation and clinical results (St. Thomas cardioplegia). The group that had the modified del Nido spent less time in the ICU and the hospital overall, need inotropic support less frequently, and experiencing fewer instances of postoperative fibrillation or flutter.
Our study showed that, no significant difference was found among group (A) & group (B) concerning ventilation duration, ICU stay & hospital stay (p > 0.05).
Additionally, a prior research found that there was no significant difference between ventilation duration, ICU stay & hospital stay (p > 0.05)[12].
In the present study, no significant difference was showed among group (A) & group (B) regarding complications (p > 0.05).
According to Kavala and Turkyilmaz, the DNC group experienced 17 obstacles whereas the BC group experienced 25 challenges[11]. None of the postoperative complications comparisons were statistically significant.
In the present research, there was no noticeable difference in in-hospital mortality among groups (A) & (B) (p > 0.05). This was consistent with Kavala and Turkyilmaz[11].
100 young patients having elective surgical correction of Fallot’s tetralogy were randomly randomized to take either Custodiol or Del Nido cardioplegic solutions in Talwar et al.[19]. A shorter time spent on mechanical ventilation, a shorter stay in the hospital and intensive care unit, improved cardiac index preservation, increased cardiac output, lower inotropic scores, and reduced troponin-I release were all associated with the first. Myocardial edema was reduced and myofibrillar architecture and glycogen storage were better preserved using electronic microscopy in the del Nido group.
-
Conclusion
We report that Modified Del Nido Cardioplegia has better postoperative chemical parameters as CK-MB, Troponin T and Lactate in mitral valve surgery on adults. DNC is a safe alternative to conventional cardioplegia and has achieved at least the same results.
-
Limitations
Our study has some limitations. First off, since it was a one- center study, other locations might have had different findings. Second, the sample size is really tiny. Moreover, Several outcomes, Lack of long-term follow-up makes it difficult to draw firm inferences about enhancements in outcomes like reduced cardiac marker levels and increased postpartum EF rates.
Abbreviations
DNCS: del Nido cardioplegia solution, EF: Ejection Fraction, SV: Stroke volume, SVV: Stroke volume variation, COP: Cardiac output, CI: Cardiac index, ASA: American Society of Anesthesiologists, CK-MB: Creatine Kinase MB, LVEF: left ventricular ejection fraction, ICU: Intensive care unite.
Funding: Nil.
Conflict of interests: Non.
References
1. Muenzer J. Overview of the mucopolysaccharidoses. Rheumatology (Oxford). 2011 Dec;50 Suppl 5:v4–12. https://doi.org/10.1093/rheumatology/ker394 PMID:22210669
2. Suárez-Guerrero JL, José P, Gómez I, Sebastian J, Flórez A, Adolfo G. Mucopolisacaridosis : características clínicas, diagnóstico y de manejo. Sociedad Chilena de Pediatría; 2015;(xx).
3. Walker R, Belani KG, Braunlin EA, Bruce IA, Hack H, Harmatz PR, et al. Anaesthesia and airway management in mucopolysaccharidosis. J Inherit Metab Dis. 2013 Mar;36(2):211–9. https://doi.org/10.1007/s10545-012-9563-1 PMID:23197104
4. Uribe A, Giugliani R. Selective Screening for Lysosomal Storage Diseases with Dried Blood Spots Collected on Filter Paper in 4,700 High-Risk Colombian Subjects. In 2013 [cited 2017 Nov 18]. p. 107–16.
5. Frawley G, Fuenzalida D, Donath S, Yaplito-Lee J, Peters H. A retrospective audit of anesthetic techniques and complications in children with mucopolysaccharidoses. Paediatr Anaesth. 2012 Aug;22(8):737–44. https://doi.org/10.1111/j.1460-9592.2012.03825.x PMID:22381044
6. Clark BM, Sprung J, Weingarten TN, Warner ME. Anesthesia for patients with mucopolysaccharidoses: comprehensive review of the literature with emphasis on airway management [Internet]. Bosn J Basic Med Sci. 2018 Feb;18(1):1–7. https://doi.org/10.17305/bjbms.2017.2201 PMID:28590232
7. Arn P, Wraith JE, Underhill L. Characterization of surgical procedures in patients with mucopolysaccharidosis type I: findings from the MPS I Registry [Internet]. J Pediatr. 2009 Jun;154(6):859–64.e3. [cited 2017 Nov 18]. https://doi.org/10.1016/j.jpeds.2008.12.024 PMID:19217123
8. James h. Díaz, MD, MHA, and Kumar G. Belani, MBBS M. Perioperative Management of Children with Mucopolysaccharido…: Anesthesia & Analgesia [Internet]. [cited 2017 Nov 18].
9. KING DH. JONES RM, BARNETT MB. Anaesthetic considerations in the mucopolysaccharidoses. Anaesthesia [Internet]. Blackwell Publishing Ltd. 1984 Feb;39(2):126–31. [cited 2017 Nov 18].
10. Sam JA, Baluch AR, Niaz RS, Lonadier L, Kaye AD. MUCOPOLYSACCHARIDOSES: ANESTHETIC CONSIDERATIONS AND CLINICAL MANIFESTATIONS.
11. Walker RW, Darowski M, Morris P, Wraith JE. Anaesthesia and mucopolysaccharidoses. A review of airway problems in children [Internet]. Anaesthesia. 1994 Dec;49(12):1078–84. [cited 2017 Nov 18]. https://doi.org/10.1111/j.1365-2044.1994.tb04360.x PMID:7864325
12. Sjøgren P, Pedersen T, Steinmetz H. Mucopolysaccharidoses and anaesthetic risks. Acta Anaesthesiol Scand [Internet]. 1987 Apr [cited 2017 Nov 18];31(3):214–8. 6576. https://doi.org/10.1111/j.1399-6576.1987.tb02553.x.
13. Mahalingam K, Janani S, Priya S, Elango EM, Sundari RM. Diagnosis of mucopolysaccharidoses: how to avoid false positives and false negatives [Internet]. Indian J Pediatr. 2004 Jan;71(1):29–32. [cited 2017 Nov 21]. https://doi.org/10.1007/BF02725652 PMID:14979382
14. Filocamo M, Morrone A. Lysosomal storage disorders: molecular basis and laboratory testing [Internet]. Hum Genomics. 2011 Mar;5(3):156–69. [cited 2017 Nov 21]. https://doi.org/10.1186/1479-7364-5-3-156 PMID:21504867
15. Gelb MH, Turecek F, Scott CR, Chamoles NA. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders [Internet]. J Inherit Metab Dis. 2006;29(2-3):397–404. [cited 2017 Nov 21]. https://doi.org/10.1007/s10545-006-0265-4 PMID:16763908
16. Marsden D, Levy H, Meikle PJ. Newborn screening of lysosomal storage disorders [Internet]. Clin Chem. 2010 Jul;56(7):1071–9. [cited 2017 Nov 21]. https://doi.org/10.1373/clinchem.2009.141622 PMID:20489136
17. Tomatsu S, Fujii T, Fukushi M, Oguma T, Shimada T, Maeda M, et al. Newborn screening and diagnosis of mucopolysaccharidoses [Internet]. Mol Genet Metab. 2013;110(1-2):42–53. [cited 2017 Nov 21]. https://doi.org/10.1016/j.ymgme.2013.06.007 PMID:23860310
18. Cimaz R, La Torre F. Mucopolysaccharidoses. Curr Rheumatol Rep. 2014 Jan;16(1):389. https://doi.org/10.1007/s11926-013-0389-0 PMID:24264718
19. Souillet G, Guffon N, Maire I, Pujol M, Taylor P, Sevin F, et al. Outcome of 27 patients with Hurler’s syndrome transplanted from either related or unrelated haematopoietic stem cell sources [Internet]. Bone Marrow Transplant. 2003 Jun;31(12):1105–17. [cited 2017 Nov 24]. https://doi.org/10.1038/sj.bmt.1704105 PMID:12796790
20. Prasad VK, Kurtzberg J. Transplant outcomes in mucopolysaccharidoses [Internet]. Semin Hematol. 2010 Jan;47(1):59–69. [cited 2017 Nov 24]. https://doi.org/10.1053/j.seminhematol.2009.10.008 PMID:20109613
21. Giugliani R, Federhen A, Rojas MV, Vieira T, Artigalás O, Pinto LL, et al. Mucopolysaccharidosis I, II, and VI: brief review and guidelines for treatment [Internet]. Genet Mol Biol. 2010 Oct;33(4):589–604. [cited 2017 Nov 24]. https://doi.org/10.1590/S1415-47572010005000093 PMID:21637564
22. Boelens JJ, Wynn RF, O’Meara A, Veys P, Bertrand Y, Souillet G, et al. Outcomes of hematopoietic stem cell transplantation for Hurler’s syndrome in Europe: a risk factor analysis for graft failure [Internet]. Bone Marrow Transplant. 2007 Aug;40(3):225–33. [cited 2017 Nov 24]. https://doi.org/10.1038/sj.bmt.1705718 PMID:17529997
23. Burton BK, Whiteman DA, Investigators HO; HOS Investigators. Incidence and timing of infusion-related reactions in patients with mucopolysaccharidosis type II (Hunter syndrome) on idursulfase therapy in the real-world setting: a perspective from the Hunter Outcome Survey (HOS) [Internet]. Mol Genet Metab. 2011 Jun;103(2):113–20. [cited 2017 Nov 24]. https://doi.org/10.1016/j.ymgme.2011.02.018 PMID:21439875
24. Tomatsu S, Giugliani R, Kubaski F, Kazuki S, Alméciga-Díaz CJ, Barrera L, et al. Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome. Drug Des Devel Ther [Internet]. 2015 Apr [cited 2017 Nov 24];9:1937.
25. Indian Society of Anaesthetists., National Institutes of Health (U.S.). PubMed Central. S, SPARC (Organization). Indian journal of anaesthesia. [Internet]. Vol. 52, Indian Journal of Anaesthesia. Indian Society of Anaesthetists; 2008 [cited 2017 Nov 18]. 453 p.
26. Pamela Arn, Chester Whitley, J. Edmond Wraith, H. Warner Webb, Lisa Underhill, Lakshmi Rangachari GFC. High rate of postoperative mortality in patients with mucopolysaccharidosis I: findings from the MPS I Registry. J Pediatr Surg [Internet]. W.B. Saunders; 2012 Mar 1 [cited 2017 Nov 18];47(3):477–84.
27. John A, Fagondes S, Schwartz I, Azevedo AC, Barrios P, Dalcin P, et al. Sleep abnormalities in untreated patients with mucopolysaccharidosis type VI. Am J Med Genet A. 2011 Jul;155A(7):1546–51. [cited 2017 Nov 24]. https://doi.org/10.1002/ajmg.a.33902 PMID:21638759
28. Braunlin EA, Harmatz PR, Scarpa M, Furlanetto B, Kampmann C, Loehr JP, et al. Cardiac disease in patients with mucopolysaccharidosis: presentation, diagnosis and management [Internet]. J Inherit Metab Dis. 2011 Dec;34(6):1183–97. [cited 2017 Nov 24]. https://doi.org/10.1007/s10545-011-9359-8 PMID:21744090
29. Misumi I, Chikazawa S, Ishitsu T, Higuchi S, Shimazu T, Ikeda C, et al. Atrioventricular block and diastolic dysfunction in a patient with Sanfilippo C [Internet]. Intern Med. 2010;49(21):2313–6. [cited 2017 Nov 24]. https://doi.org/10.2169/internalmedicine.49.4210 PMID:21048366
30. Ingelmo PM, Parini R, Grimaldi M, Mauri F, Romagnoli M, Tagliabue G, et al. Multidetector computed tomography (MDCT) for preoperative airway assessment in children with mucopolysaccharidoses [Internet]. Minerva Anestesiol. 2011 Aug;77(8):774–80. [cited 2017 Nov 24]. PMID:21730924
31. Muhlebach MS, Shaffer CB, Georges L, Abode K, Muenzer J. Bronchoscopy and airway management in patients with mucopolysaccharidoses (MPS). Pediatr Pulmonol. 2013 Jun;48(6):601–7. https://doi.org/10.1002/ppul.22629 PMID:22949390
32. Walker RW. The laryngeal mask airway in the difficult paediatric airway: an assessment of positioning and use in fibreoptic intubation. Paediatr Anaesth. 2000;10(1):53–8. https://doi.org/10.1046/j.1460-9592.2000.00425.x PMID:10632910
33. Walker RW, Colovic V, Robinson DN, Dearlove OR. Postobstructive pulmonary oedema during anaesthesia in children with mucopolysaccharidoses [Internet]. Paediatr Anaesth. 2003 Jun;13(5):441–7. [cited 2017 Nov 25]. https://doi.org/10.1046/j.1460-9592.2003.00969.x PMID:12791120
34. Kirkpatrick K, Ellwood J, Walker RW. Mucopolysaccharidosis type I (Hurler syndrome) and anesthesia: the impact of bone marrow transplantation, enzyme replacement therapy, and fiberoptic intubation on airway management. Paediatr Anaesth. 2012 Aug;22(8):745–51. https://doi.org/10.1111/j.1460-9592.2012.03897.x PMID:22672476
35. Cohen MA, Stuart GM. Delivery of anesthesia for children with Mucopolysaccharidosis Type III (Sanfilippo syndrome): a review of 86 anesthetics. Paediatr Anaesth. 2017 Apr;27(4):363–9. https://doi.org/10.1111/pan.13075 PMID:28098417
36. Kamata M, McKee C, Truxal KV, Flanigan KM, McBride KL, Aylward SC, et al. General anesthesia with a native airway for patients with mucopolysaccharidosis type III. Paediatr Anaesth. 2017 Apr;27(4):370–6. https://doi.org/10.1111/pan.13108 PMID:28181359

 ORCID
ORCID