Warda Demerdash Khalifa Ali1,*, Sabah Nagiub Barsoom Ayoub2
Recibido: 23-06-2023
Aceptado: 06-09-2023
©2024 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 53 Núm. 2 pp. 124-129|https://doi.org/10.25237/revchilanestv53n2-07
PDF|ePub|RIS
Velocidad de recuperación de anestesia general. Efectos de estimulación auditiva versus táctil
Abstract
Background and aim: Time for recovery from anaesthesia varies depending on genetic factors, comorbidities, and age. Prolonged recovery delays return to baseline and safe functioning, and increases costs due to more time spent in post-anaesthesia recovery units. The present study aimed to compare the effect of tactile and auditory stimulations on patients’ recovery from general anaesthesia. Materials and Methods: This randomised, double blinded, two-centre, clinical trial was conducted at King Abdullah Bin Abdul-Aziz University Hospital, Riyadh and King Salman Specialist Hospital, Hail, KSA, from January through August, 2021. Standard monitoring was maintained throughout surgery. Bi-spectral Index (BIS) was monitored through electrodes applied on the patient’s forehead. Perfusion index was assessed using MASiMO, (MASiMO, model;Root, designed by MASiMO in California; assembled in Mexico, Masimo corporation, 52 discovery, Irvine, CA92618 USA). The disposable sensors and pulse oximetry (RD rainbow SET) was connected. During the maintenance phase, patients were allowed to breathe spontaneously sevoflurane (2%, end-tidal sevoflurane concentration was measured, and MAC was adjusted to maintain the BIS value < 60, and the mean blood pressure and heart rate within 20% up and down of baseline values.) all through the surgery time. With start of skin suturing sevoflurane was discontinued & 0.25 pg/kg of fentanyl was given. Throughout the surgery, sevoflurane was discontinued and 0.25 pg/kg of fentanyl was given (total range: 12.5-23.5 pg). Results: Group T patients had significantly shorter time to first eye opening (272.24 ± 7.37 versus 333.68 ± 46.82 seconds, p < 0.001), shorter LMA removal time (322.82 ± 7.61 versus 380.82 ± 19.31 seconds, p < 0.001) and shorter PACU time (36.82 ± 3.78 versus 43.16 ± 4.34 minutes, p < 0.001) when compared group A patients. Also, patients in the T group needed significantly shorter time to reach BIS > 60 (196.24 ± 27.13 versus 208.32 ± 27.24 seconds, p = 0.02). Conclusion:Tactile stimulation is associated with shorter time to recovery from general anaesthesia as compared to auditory stimulation.
Resumen
Antecedentes y Objetivo: El tiempo de recuperación de la anestesia varía en función de factores genéticos, comorbilidades y edad. Una recuperación prolongada retrasa el retorno a la situación basal y al funcionamiento seguro, y aumenta los costos debido al mayor tiempo que se pasa en las unidades de recuperación postanestésica. El presente estudio tenía como objetivo comparar el efecto de las estimulaciones táctiles y auditivas en la recuperación de los pacientes tras la anestesia general. Pacientes y Métodos: En este ensayo clínico aleatorizado, doble ciego y bicéntrico participaron 100 mujeres adultas ASA I-II sometidas a cirugía electiva ambulatoria bajo anestesia general. Las pacientes se distribuyeron aleatoriamente en dos grupos de 50 cada uno. Las intervenciones utilizadas para la recuperación incluyeron estimulación táctil (grupo T) o estimulación auditiva (grupo A). Los parámetros de resultado fueron el tiempo transcurrido hasta la primera apertura de los ojos, el tiempo transcurrido hasta alcanzar un valor del índice biespectral (BIS) > 60 y un valor del índice de perfusión (PI) < 5, el tiempo de retirada de la mascarilla laríngea (LMA) tras la interrupción de la anestesia, la prueba de concentración de memoria de orientación breve (SOMCT) medida a los 30, 45, 60 y 90 minutos tras la retirada de la LMA por un registrador ciego. Otros parámetros de resultado fueron el uso de fármacos analgésicos o antieméticos en la unidad de cuidados posanestésicos (UPA) y la duración de la estancia en la UPA. Resultados: Los pacientes del grupo T tuvieron un tiempo significativamente menor hasta la primera apertura del ojo (272,24 ± 7,37 frente a 333,68 ± 46,82 segundos, p < 0,001), un tiempo menor de retirada de la ML (322,82 ± frente a 380,82 ± 19,31 segundos, p < 0,001) y menor tiempo en la PACU (36,82 ± 3,78 frente a 43,16 ± 4,34 minutos, p < 0,001) en comparación con los pacientes del grupo A. Además, los pacientes del grupo T necesitaron un tiempo significativamente menor para alcanzar un BIS > 60 (196,24 ± 27,13 frente a 208,32 ± 27,24 segundos, p=0,02).
-
Introduction
General anaesthesia comprises amnesia, hypnosis, analgesia, and areflexia[1]. Anaesthesia acts on the brain network multimodally to alter network connectivity.
Sequential activation of consciousness, connectedness to the environment, and responsiveness is an important process for smooth and uneventful emergence from anaesthesia[2]-[4]. Time for recovery from anaesthesia varies depending on genetic factors, comorbidities, and age. Prolonged recovery delays return to baseline and safe functioning, and increases costs due to more time spent in post-anaesthesia recovery units[5],[6].
Emergence from anaesthesia and restoring consciousness was once considered a completely passive process achieved when the effect-site concentration of the anaesthetic agent decreases below its therapeutic range[7]. However, recently, emergence from anaesthesia is regarded as a controllable and active process in which multiple biochemical mediators e.g. acetylcholine, norepinephrine, orexin/hypocretin, dopamine and adenosine are involved[8].
During this process, providing additional stimulation can facilitate the return of consciousness[9]. Tactile stimulation is known to be suppressed by isoflurane and propofol at different hypnotic levels[10]. Auditory word stimulation tested during 1% and 2% sevoflurane anaesthesia and a dose-dependent suppression of auditory blood oxygen level-dependent (BOLD) activation have been found, suggesting limited processing and memory of the presented auditory stimulus[11],[12].
The present study aimed to compare the effect of tactile and auditory stimulations on patients’ recovery from general anaesthesia.
-
Materials and Methods
This randomised, double blinded, two-centre, clinical trial was conducted at King Abdullah Bin Abdul-Aziz University Hospital and King Salman Specialist Hospital from January through August, 2021. The study protocol was approved by the Institutional Review Board of the two centres and all patients provided written informed consent before enrolment.
The study included 100 ASA I-II adult females undergoing elective ambulatory surgery under general anaesthesia. Patients were excluded if they have hearing problems, neurological, cardiovascular, hepatic or renal dysfunction, mental retardation, body mass index > 30 kg/m2, history of alcohol or drug dependence, or medication affecting the central nervous system.
Patients were randomly allocated into two groups of 50 each using sealed envelopes opened by the attending staff nurse at the end of surgery just before emergence phase to perform the required intervention for the patient.
-
Anaesthetic protocol
At the preoperative area, patients were lightly pre-medicated with 2 mg midazolam after IV cannula insertion. Preemptive analgesia (paracetamol 15 mg/kg) and nausea prophylaxis (granisetron 1 mg) were achieved. Standard monitoring was maintained throughout surgery. Bi-spectral Index (BIS) was monitored through electrodes applied on the patient’s forehead. Perfusion index was assessed using MASiMO disposable. Forced-air warming was applied to the upper and lower body. Preoxygenation with 100% O2 was achieved via a face mask and then an IV bolus of 1-1.5 pg/kg fentanyl and 2 to 2.5 mg/ kg propofol were administered to induce anaesthesia was given. A Pro-seal laryngeal mask airway (Laryngeal Mask Company Limited, USA) was inserted orally into the larynx to complete the induction phase.
During the maintenance phase, participants were allowed to spontaneously breathe sevoflurane 2%. End-tidal sevoflu- rane concentration was measured, and minimum alveolar concentration (MAC) was adjusted to maintain the BIS value < 60, the mean blood pressure and heart rate within 20% of baseline values. Throughout the surgery, sevoflurane was discontinued and 0.25 pg/kg of fentanyl was given (total range: 12.5-23.5 pg). A curtain was then put in between the patient, the anaesthesiologist and a blinded recorder who is not a physician. An assistant anaesthesiologist monitored the patient throughout period of recovery without any intervention unless required as an emergency measure. After discontinuation of sevoflurane, patients were allowed to emerge naturally and unperturbed. With an allowance of only a small amount of noise from the monitoring equipment, the room will be otherwise kept quiet.
-
Study interventions
A staff nurse who opened the randomization envelopes performed the interventions under investigation including:
• Tactile stimulation group (T group): At room air temperature, a wet gauze was rubbed gently on the patient face
and back of the neck for 10s. Then face was dried by another dry gauze. This was repeated until patients opened their eyes.
• Auditory stimulation group (A group): A noise-cancelling headphone was placed over the patient’s ears and connected to the voice recording device. The pre-recorded message by patient name was played and repeated at 10s intervals, with the volume set to a normal speech level. It was repeated until the subjects opened their eyes.
Patients with BIS value > 60, spontaneous eye opening and adequate spontaneous breathing were transferred to postanaesthesia care unit (PACU). Post-operative analgesia was achieved by 0.5 mg/kg intravenous boluses of meperidine. Patients were only discharged from PACU when they were alert, oriented, conversant and cooperative, their vital signs were stable for at least 30 minutes and can sit up without dizziness, nausea or intolerable pain (Visual analogue scale (VAS) < 3).
-
Study measurements
Recoded data included demographic data, operative data (duration of surgery, duration of anaesthesia, intraoperative variables). Outcome parameters included time to first eye-opening; time to reach BIS value > 60 and perfusion index (PI) value < 5, laryngeal mask airway (LMA) removal time after discontinuation of anesthesia, Short Orientation Memory Concentration Test (SOMCT) 13 measured at 30, 45, 60 and 90 minutes after LMA removal by the blinded recorder. Other outcome parameters were use of analgesic or antiemetic drugs in the PACU and length of PACU stay.
Data were recorded and analyzed using statistical package for social sciences (SPSS) version 21.0 (Chicago, Illinois, USA). Quantitative data expressed as mean ± standard deviation. Qualitative data expressed as count and percentage. The independent samples t-test was used to compare between means in the two groups. Chi- square test was used to compare proportions between two qualitative parameters. P < 0.05 was considered significant.
Table 1. Demographic and surgical data of the studied groups
| Group T n = 50 | Group A n = 50 | p value | |
| Age (years) mean ± SD | 32.14 ± 6.66 | 31.32 ± 5.85 | 0.26 |
| Height (cm) mean ± SD | 157.80 ± 2.92 | 157.88 ± 2.75 | 0.45 |
| Weight (kg) mean ± SD | 71.92 ± 6.14 | 73.02 ± 8.47 | 0.23 |
| ASA I/II n | 31/19 | 28/22 | 0.54 |
| Surgery duration (minutes) mean ± SD | 61.16 ± 9.9 | 59.17 ± 10.3 | 0.24 |
| Anaesthesia duration (minutes) mean ± SD | 82.60 ± 10.6 | 82.44 ± 10.4 | 0.47 |
ASA: American society of anaesthesiologists.
Table 2. Recovery measures in the studied groups
| Group T n = 50 | Group A n = 50 | p value | |
| MAC of ET sevoflurane at recovery (%) mean ± SD | 0.124 ± 0.28 | 0.116 ± 0.12 | 0.428 |
| Temperature at time of recovery (°C) mean ± SD | 35.580 ± 4.12 | 35.48 ± 0.30 | 0.160 |
| Time to reach BIS > 60 (seconds) mean ± SD | 196.24 ± 27.13 | 208.32 ± 27.24 | O.O2 |
| Time to reach PI < 5 (seconds) mean ± SD | 181.98 ± 20.41 | 179.76 ± 16.38 | 0.277 |
| Time to first eye opening (seconds) mean ± SD | 272.24 ± 7.37 | 333.68 ± 46.82 | < 0.001 |
| LMA removal time (seconds) mean ± SD | 322.82 ± 7.61 | 380.82± 19.31 | < 0.001 |
| PACU stay time (minutes) mean ± SD | 36.82 ± 3.78 | 43.16 ± 4.34 | < 0.0001 |
BIS: Bi-spectral index; ET: end tidal; LMA: Laryngeal mask airway; MAC: Minimum alveolar concentration; PACU: Post anaesthesia care unit; PI: Perfusion index.
-
Results
Comparison between the studied groups regarding the baseline data revealed no statistically significant differences (Table 1). Regarding the recovery measures in the studied groups, we found that group T patients had significantly shorter time to first eye opening (272.24 ± 7.37 versus 333.68 ± 46.82 seconds, p < 0.001), shorter LMA removal time (322.82± 7.61 versus 380.82 ± 19.31 seconds, p < 0.001) and shorter PACU time (36.82 ± 3.78 versus 43.16 ± 4.34 minutes, p < 0.001) when compared group A patients. Also, patients in the T group needed significantly shorter time to reach BIS > 60 (196.24 ± 27.13 versus 208.32 ± 27.24 seconds, p = 0.02) (Table 2).
No significant differences were found between the studied groups regarding BIS or heart rate measurements at different intervals (Table 3). Moreover, no significant differences were found between the studied groups regarding mean blood pressure (Figure 1). During the PACU period, the need of postoperative analgesia was 64% in group T patients versus 52% in group A patients (p = 0.086) while need for antiemetics was 9% and 13% in groups T and A respectively (p = 0.37) (Figure 2). The SOMCT score was significantly lower in group T patients at 30 min after the recovery in PACU (Figure 3).
Table 3. BIS and heart rate readings in the studied groups
| Group T n = 50 | Group A n = 50 | p value | |
| BIS mean ± SD | |||
| Before LMA insertion | 43.40 ± 4.37 | 43.02 ± 4.72 | 0.34 |
| At start of surgery | 34.44 ± 3.74 | 35.34 ± 3.77 | 0.12 |
| 30 min after start of surgery | 32.04 ± 3.01 | 32.36 ± 2.87 | 0.3 |
| After discontinuation of sevoflurane | 64.06 ± 3.61 | 63.60 ± 5.23 | 0.31 |
| At LMA removal | 72.02 ± 5.46 | 71.54 ± 5.53 | 0.33 |
| Heart Rate (beat/min.) mean ± SD | |||
| Baseline | 88.120 ± 8.56 | 86.62 ± 10.41 | 0.22 |
| At discontinuation of anaesthesia | 74.20 ± 10.01 | 72.04 ± 8.61 | 0.13 |
| At BIS of 60 | 97.80 ± 12.43 | 100.24 ±12 | 0.16 |
| At first eye opening | 118.46 ± 10.24 | 117.88 ± 10.28 | 0.39 |
| At LMA removal | 119.42 ± 9.01 | 119.82 ± 9.96 | 0.42 |
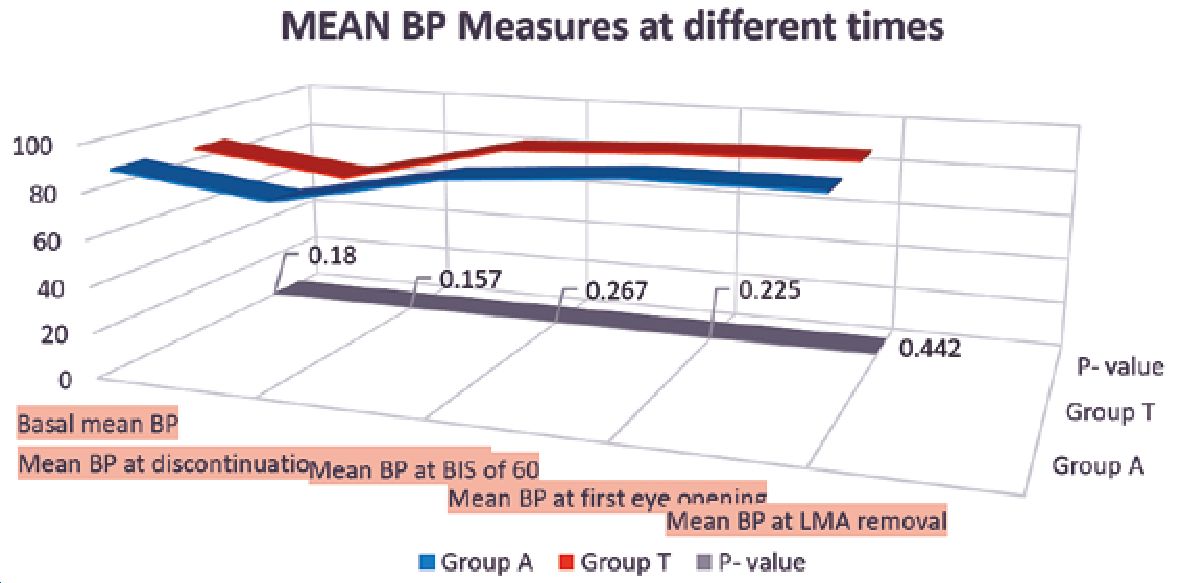
Figure 1. Mean values of non- invasive blood pressure at different time during the case in both groups. At the bottom of the figure grey straight line is the p value at that time which shows there was no significant difference throughout.
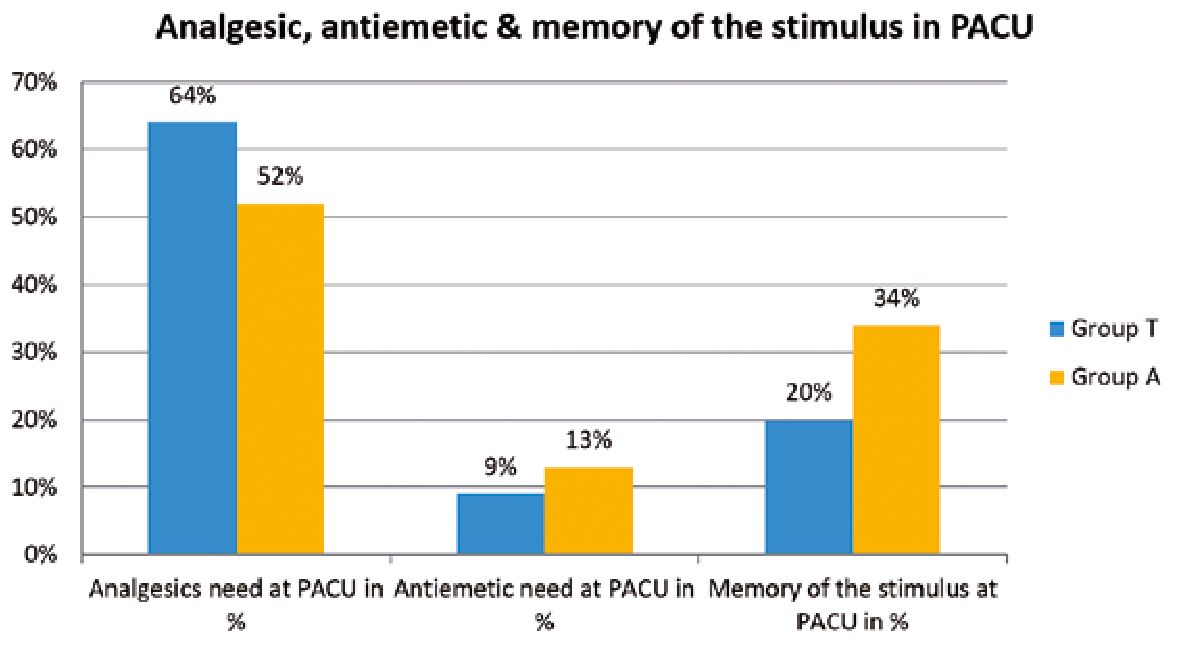
Figure 2. Requirements of analgesics, antiemetics and the percentage of patients remembering the stimulus given to them at recovery. Blue bars are the tactile group and yellow bars are auditory group.
-
Discussion
In this study, we aimed to compare the efficacy of auditory and tactile stimuli on the speed of recovery from general anaesthesia. Our results showed that the time to eye opening after discontinuation of anaesthesia was significantly shorter in the tactile group than in the auditory group. Moreover, patients in the tactile group had shorter LMA removal time and PACU time. Also, patients in the T group needed significantly shorter time to reach BIS > 60.
The advantages of tactile over auditory stimuli during emergence from general anaesthesia are attributed to the different mechanisms of central processing of these stimuli during postanaesthetic recovery. Anaesthetic actions on nuclei involved in arousal and the sleep/wake cycle constitute on/off switches whose effects are mediated through actions in the cortico-tha- lamic network. Anaesthetic modulation of cortical responses to auditory stimuli causes reduction and slowing of field potentials recorded at the surface by several classes of anaesthetic agents at surgical (i.e., higher than just-hypnotic) doses. Moreover, cortical responses to verbal stimuli are maintained in primary auditory cortex, but disrupted in higher order cortex under deep level of anaesthesia. Evidence indicates that memory formation is extremely sensitive to anaesthesia, with concentrations suppressing recall approximately one half those causing loss of consciousness. In humans, the incidence of recall under anaesthesia is exceedingly low[14].
Post-anaesthetic recovery would be an extended process rather than a single point, commencing with return of responsiveness and concluding with return of executive function. Studies hypothesized that executive function would be the last to recover because there is evidence that neurologic recovery from general anaesthesia occurs in a caudal-to-rostral direc- tion[15],[16].
The superiority of tactile over verbal stimulation may be also explained by neurochemical factors. Tactile stimulation was reported to increase cortical acetylcholine in rats[17]. Elevation of acetylcholine is associated with accelerated recovery from general anaesthesia[18]. Moreover, tactile stimulation was found to enhance dopamine 1 receptors expression in rats[19] which can promote emergence from general anaesthesia[20].
In conclusion, we observed that tactile stimulus is very much helpful in speeding up the recovery phase from general anaesthesia. It makes patient more conscious and alert than auditory stimulus in less time. This simple manoeuvre can be incorporated in daily practice to increase the operating room patient turn over and minimizing PACU stay.
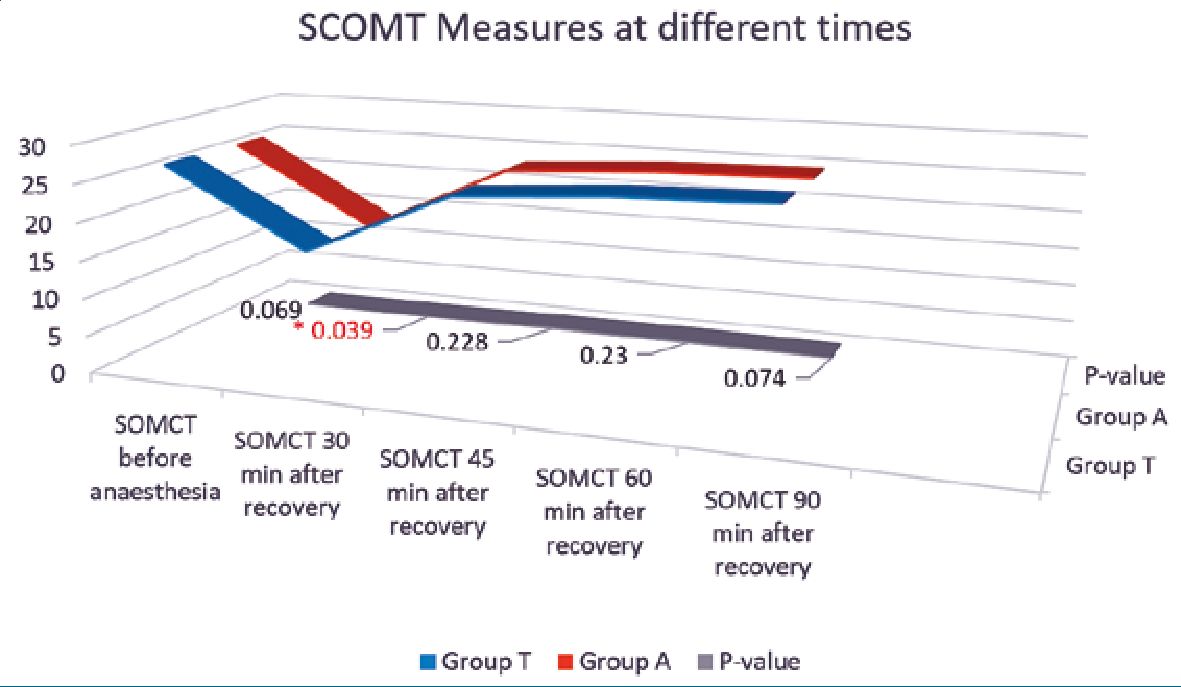
Figure 3. SCOMT values for both study group at different time and p value at the bottom of graph. Significant low value at 30 minutes after the recovery in PACU. The SCOMT value was less than 20 in both the group at that time and recovered just in 15 minutes. The difference was measured with the normal SCOMT value before induction of anaesthesia.
Conflict: Authors state no conflict of interest.
Funding: The research is self-funded from the authors.
-
References
1. Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010 Dec;363(27):2638–50. https://doi.org/10.1056/NEJMra0808281 PMID:21190458
2. Kushikata T, Hirota K. Mechanisms of Anesthetic Emergence: Evidence for Active Reanimation. Current Anesthesiology Reports. 2014/03/01 2014;4(1):49-56. https://doi.org/10.1007/s40140-013-0045-2.
3. Lee U, Müller M, Noh GJ, Choi B, Mashour GA. Dissociable network properties of anesthetic state transitions. Anesthesiology. 2011 Apr;114(4):872–81. https://doi.org/10.1097/ALN.0b013e31821102c9 PMID:21383615
4. Sanders RD, Tononi G, Laureys S, Sleigh JW, Warner DS. Unresponsiveness ≠ unconsciousness. Anesthesiology. 2012 Apr;116(4):946–59. https://doi.org/10.1097/ALN.0b013e318249d0a7 PMID:22314293
5. Chen X, Zhao M, White PF, et al. The recovery of cognitive function after general anesthesia in elderly patients: a comparison of desflurane and sevoflurane. Anesth Analg. Dec 2001;93(6):1489-94, table of contents. https://doi.org/10.1097/00000539-200112000-00029.
6. Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011 Oct;115(4):791–803. https://doi.org/10.1097/ALN.0b013e31822e92e5 PMID:21934407
7. Antognini JF, Buonocore MH, Disbrow EA, Carstens E. Isoflurane anesthesia blunts cerebral responses to noxious and innocuous stimuli: a fMRI study. Life Sci. 1997;61(24):PL 349-54. https://doi.org/10.1016/S0024-3205(97)00960-0.
8. Kelz MB, García PS, Mashour GA, Solt K. Escape From Oblivion: Neural Mechanisms of Emergence From General Anesthesia. Anesth Analg. 2019 Apr;128(4):726–36. https://doi.org/10.1213/ANE.0000000000004006 PMID:30883418
9. Seo JH, Goo EK, Song IA, Park SH, Park HP, Jeon YT, et al. Influence of a modified propofol equilibration rate constant (k(e0)) on the effect-site concentration at loss and recovery of consciousness with the Marsh model. Anaesthesia. 2013 Dec;68(12):1232–8. https://doi.org/10.1111/anae.12419 PMID:24032636
10. Iwakiri H, Nishihara N, Nagata O, Matsukawa T, Ozaki M, Sessler DI. Individual effect-site concentrations of propofol are similar at loss of consciousness and at awakening. Anesth Analg. 2005 Jan;100(1):107–10. https://doi.org/10.1213/01.ANE.0000139358.15909.EA PMID:15616062
11. Bonhomme V, Fiset P, Meuret P, Backman S, Plourde G, Paus T, et al. Propofol anesthesia and cerebral blood flow changes elicited by vibrotactile stimulation: a positron emission tomography study. J Neurophysiol. 2001 Mar;85(3):1299–308. https://doi.org/10.1152/jn.2001.85.3.1299 PMID:11247998
12. Kerssens C, Hamann S, Peltier S, Hu XP, Byas-Smith MG, Sebel PS. Attenuated brain response to auditory word stimulation with sevoflurane: a functional magnetic resonance imaging study in humans. Anesthesiology. 2005 Jul;103(1):11–9. https://doi.org/10.1097/00000542-200507000-00006 PMID:15983451
13. Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983 Jun;140(6):734–9. https://doi.org/10.1176/ajp.140.6.734 PMID:6846631
14. Raz A, Grady SM, Krause BM, Uhlrich DJ, Manning KA, Banks MI. Preferential effect of isoflurane on top-down vs. bottom-up pathways in sensory cortex. Front Syst Neurosci. 2014 Oct;8:191. https://doi.org/10.3389/fnsys.2014.00191 PMID:25339873
15. Långsjö JW, Alkire MT, Kaskinoro K, Hayama H, Maksimow A, Kaisti KK, et al. Returning from oblivion: imaging the neural core of consciousness. J Neurosci. 2012 Apr;32(14):4935–43. https://doi.org/10.1523/JNEUROSCI.4962-11.2012 PMID:22492049
16. Reshef ER, Schiff ND, Brown EN. A Neurologic Examination for Anesthesiologists: Assessing Arousal Level during Induction, Maintenance, and Emergence. Anesthesiology. 2019 Mar;130(3):462–71. https://doi.org/10.1097/ALN.0000000000002559 PMID:30664547
17. Himmelheber AM, Fadel J, Sarter M, Bruno JP. Effects of local cholinesterase inhibition on acetylcholine release assessed simultaneously in prefrontal and frontoparietal cortex. Neuroscience. 1998 Oct;86(3):949–57. https://doi.org/10.1016/S0306-4522(98)00097-9 PMID:9692730
18. Hambrecht-Wiedbusch VS, Li D, Mashour GA. Paradoxical Emergence: Administration of Subanesthetic Ketamine during Isoflurane Anesthesia Induces Burst Suppression but Accelerates Recovery. Anesthesiology. 2017 Mar;126(3):482–94. https://doi.org/10.1097/ALN.0000000000001512 PMID:28099246
19. Zhang M, Cai JX. Neonatal tactile stimulation enhances spatial working memory, prefrontal long-term potentiation, and D1 receptor activation in adult rats. Neurobiol Learn Mem. 2008 May;89(4):397–406. https://doi.org/10.1016/j.nlm.2007.10.010 PMID:18077190
20. Ongini E, Caporali MG, Massotti M. Stimulation of dopamine D-1 receptors by SKF 38393 induces EEG desynchronization and behavioral arousal. Life Sci. 1985 Dec;37(24):2327–33. https://doi.org/10.1016/0024-3205(85)90025-6 PMID:3877855

 ORCID
ORCID