Leonel Santiago Vega Useche1,5, Camilo Antonio Vega Useche2,5, María José Erazo Muñoz3, Christian Vladimir Guauque Marcelo4
Recibido: 13-08-2025
Aceptado: 23-09-2025
©2026 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 55 Núm. 1 pp. 42-44|https://doi.org/10.25237/revchilanestv55n1-06
PDF|ePub|RIS
Suzetrigina: ¿es la nueva arma de la analgesia multimodal?
Abstract
The opioid crisis remains one of the most pressing public health challenges worldwide. The risks of misuse, addiction, and overdose associated with opioids have led clinicians and researchers to pursue safer alternatives for acute pain management. Suzetrigine (VX-548), recently approved by the U.S. FDA, is a novel non-opioid analgesic that selectively inhibits the NaV1.8 sodium channel, offering a new peripheral mechanism for pain control. To summarize the current evidence on the pharmacological properties, clinical efficacy, safety profile, and challenges related to the use of Suzetrigine in acute pain treatment. Suzetrigine binds specifically to the voltage-sensing domain 2 (VSD2) of NaV1.8, inhibiting sodium influx and neuronal excitability. It exhibits high plasma protein binding, extensive tissue distribution, and a pharmacokinetic profile supporting twice-daily dosing. Clinical trials have shown statistically significant pain reduction following abdominoplasty and bunionectomy compared to placebo, though not powered to establish superiority over opioids. Adverse events were mild and non-opioid-like. Limitations include its high cost, uncertain role in chronic pain, and limited long-term data. Observational studies suggest its utility in multimodal analgesia, particularly among opioid-naive patients or those with opioid use disorder. Suzetrigine is a promising addition to the multimodal analgesic arsenal. Its peripheral selectivity, tolerability, and potential to reduce opioid reliance position it as an innovative option in acute pain management. Further studies should assess long-term safety, pharmacoeconomic impact, and the influence of pharmacogenomics to optimize its use in diverse patient populations.
Resumen
La crisis de los opioides sigue siendo uno de los desafíos más urgentes de la salud pública a nivel mundial. Los riesgos de uso indebido, adicción y sobredosis asociados con los opioides han llevado a clínicos e investigadores a buscar alternativas más seguras para el manejo del dolor agudo. Suzetrigina (VX-548), recientemente aprobada por la FDA de los Estados Unidos, es un novedoso analgésico no opioide que inhibe selectivamente el canal de sodio NaV1.8, ofreciendo un nuevo mecanismo periférico para el control del dolor. Resumir la evidencia actual sobre las propiedades farmacológicas, eficacia clínica, perfil de seguridad y desafíos relacionados con el uso de la suzetrigina en el tratamiento del dolor agudo. La suzetrigina se une específicamente al dominio sensor de voltaje 2 (VSD2) del canal NaV1.8, inhibiendo el influjo de sodio y la excitabilidad neuronal. Presenta una elevada unión a proteínas plasmáticas, amplia distribución tisular y un perfil farmacocinético que respalda la administración dos veces al día. Los ensayos clínicos han demostrado una reducción del dolor estadísticamente significativa tras abdominoplastia y bunionectomía en comparación con placebo, aunque sin potencia suficiente para establecer superioridad frente a opioides. Los eventos adversos fueron leves y no similares a los opioides. Sus limitaciones incluyen el alto costo, un papel incierto en el dolor crónico y la escasa evidencia a largo plazo. Estudios observacionales sugieren su utilidad en la analgesia multimodal, en particular en pacientes sin exposición previa a opioides o con trastorno por consumo de opioides. La suzetrigina representa una incorporación prometedora al arsenal analgésico multimodal. Su selectividad periférica, buena tolerabilidad y potencial para reducir la dependencia de opioides la posicionan como una opción innovadora en el manejo del dolor agudo. Se requieren estudios adicionales para evaluar su seguridad a largo plazo, impacto farmacoeconómico y la influencia de la farmacogenómica con el fin de optimizar su uso en poblaciones diversas de pacientes.
The opioid crisis continues to pose one of the most serious public health challenges of our time. The inappropriate use, addiction, and overdose related to opioids have triggered global concern, especially among pain medicine specialists who are responsible for the daily prescription of these agents to patients in acute and chronic settings. Since the early 2000s, opioid use disorder has increased dramatically, with an estimated 2.5 million individuals affected in the United States by 2015, and opioid-related deaths surpassing those caused by heroin, accounting for approximately 37% of all drug overdose fatalities in that year alone[1].
In response to this crisis, pain specialists, researchers, and pharmaceutical companies have pursued alternative therapeutic strategies that can effectively address moderate-to-severe pain without the inherent risks of addiction or dependency. After more than two decades of investigation, a significant milestone was reached in early 2025 with the U.S. Food and Drug Administration (FDA) approval of VX-548, also known as Su- zetrigine, a non-opioid analgesic with a novel mechanism of action. Commercially marketed as Journavx, this agent represents a potential paradigm shift in the treatment of acute pain[2].
Suzetrigine acts by binding to a specific motif on the second voltage-sensing domain (VSD2) of NaV1.8, particularly the KKGS amino acid sequence, a unique site not conserved in other sodium channels. This selective interaction stabilizes the channel in its closed state, preventing sodium influx, reducing neuronal excitability, and attenuating pain transmission from peripheral tissues to the central nervous system[2],[3]. Pharmacokinetic studies indicate that Suzetrigine exhibits high plasma protein binding (approximately 99%), and its primary active metabolite, M6-SUZ, shows 96% binding. After oral administration, the peak plasma concentration (Tmax) of Suzetrigine is achieved at 3 hours, while M6-SUZ peaks around 10 hours. The half-life of the parent drug is 23.6 hours, and that of M6- SUZ is about 33 hours, supporting a twice-daily dosing sched- ule[4],[5].
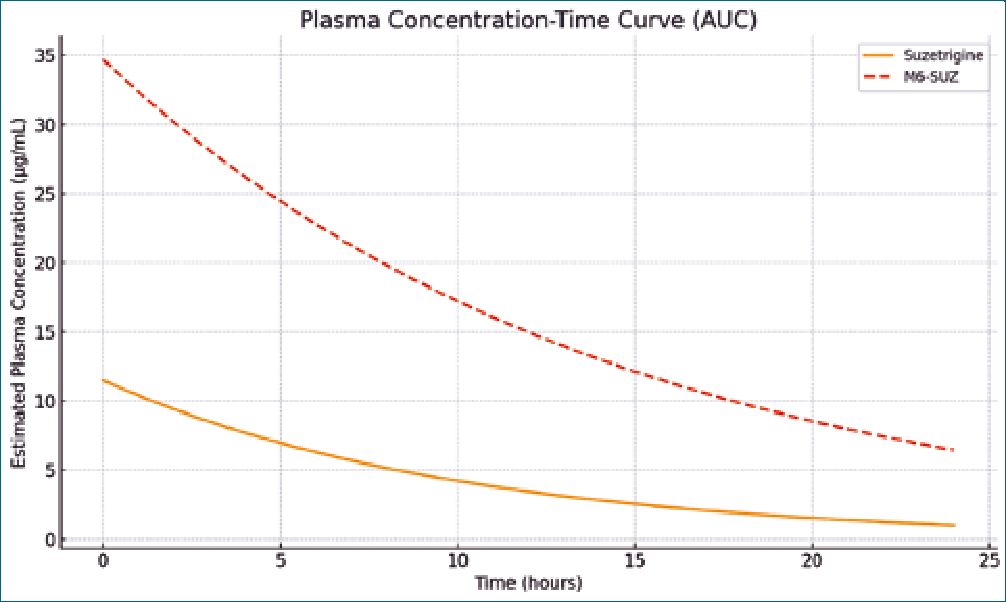
Suzetrigine has a large apparent volume of distribution (~495 L) and an oral clearance of 13.9 L/h, indicating widespread tissue distribution without significant accumulation in red blood cells. Elimination is primarily fecal (50%, with 9% unchanged drug) and renal (44%, mostly as metabolites)[4]. These pharmacokinetic parameters have supported the development of a dosing regimen that allows for sustained analgesic effects, as reflected by the area under the curve (AUC) values of 11.5 pg-h/mL for Suzetrigine and 34.7 pg-h/mL for M6-SUZ. (Figure 1)[5].
Regarding drug interactions, potent CYP3A inhibitors such as ketoconazole can increase Suzetrigine exposure by nearly 4.8-fold. Moderate inhibitors like fluconazole raise AUC by 1.5- fold, while strong inducers such as Rifampin reduce exposure by 93%. These findings underline the necessity of careful consideration of co-administered medications[5].
Clinical trials have confirmed that Suzetrigine exhibits a favorable safety profile, with the most frequently reported side effects being pruritus, mild skin rashes, and muscle spasms. Importantly, no cases of respiratory depression, euphoria, or other opioid-like adverse events were documented[6]. However, its clinical effectiveness remains under discussion. While phase II and III trials demonstrated statistically significant reductions in postoperative pain compared to placebo-particularly in patients undergoing abdominoplasty and bunionectomy who had no prior exposure to chronic opioids or NSAIDs-the comparative strength of these trials against traditional opioid regimens has been questioned[7],[8],[9]. The dosing scheme employed in these studies consisted of an initial 100 mg loading dose followed by 50 mg every 12 hours for 48 hours and was compared to Acetaminophen/Hydrocodone (325 mg/5 mg) given every 6 hours over the same period[8],[9]. Despite promising outcomes, the trials were not powered for superiority over opioid combinations, and thus broader clinical implementation should be approached with careful consideration.
Complementing these controlled trials, observational studies and single-arm phase III research have further supported the utility of Suzetrigine. In a study involving post-surgical patients reporting pain scores > 4 on the Numerical Rating Scale, over 76% received Suzetrigine in conjunction with Acetaminophen and Ibuprofen, aligning with current multimodal analgesia strategies. Notably, 80% of participants expressed positive perceptions regarding analgesic efficacy, and only 2% experienced mild adverse events such as headache, constipation, or nau- sea[10]. These findings highlight both its potential as a primary agent in moderate-to-severe pain and its compatibility with adjunctive pharmacologic regimens.
Figure 1. Estimated plasma concentration-time profiles for Suzetrigine and its active metabolite M6-SUZ over a 24-hour dosing period.
The inclusion of Suzetrigine into clinical practice resonates with the growing emphasis on multimodal and opioid-free analgesia protocols. In surgical contexts, especially within Enhanced Recovery After Surgery (ERAS) programs, this agent offers a novel non-opioid mechanism that can complement traditional agents, such as acetaminophen or NSAIDs, which act through different pathways. The combination of pharmacologic diversity and peripheral specificity may yield improved pain control while mitigating the risk of adverse outcomes associated with opioid use. In individuals with a history of opioid use disorder, in particular, Suzetrigine represents a promising option to avoid re-exposure and the consequent risk of relapse[1],[11].
Nevertheless, several limitations merit attention. First, while the drug is well-tolerated and pharmacologically promising, its current cost poses a major barrier to universal accessibility. Each 50 mg dose is priced at approximately USD $15, far exceeding the cost of standard analgesics currently in use[7]. In low- and middle-income settings, or within healthcare systems constrained by budgetary limitations, this could severely limit the drug’s uptake. Future investigations must rigorously assess the economic viability of incorporating Suzetrigine into multimodal regimens, ideally through cost-utility and cost-effectiveness analyses that consider both direct and indirect healthcare savings. If this agent can reduce opioid consumption, shorten hospital stays, or lower readmission rates, such benefits may offset its higher up-front cost.
Second, the long-term efficacy of Suzetrigine in chronic pain management remains unknown. Although the drug shows efficacy in acute postoperative settings, its role in subacute and chronic pain has yet to be defined. Likewise, more robust comparative studies are needed to better determine its place in the analgesic hierarchy. Pharmacogenomic factors may also influence individual responses to Nav1.8 inhibition, and future research could explore genetic polymorphisms that affect efficacy, tolerability, or metabolism[3],[12].
From this perspective, Suzetrigine opens a valuable avenue for further innovation in peripheral sodium channel-targeting analgesics. While Nav1.8 has now been validated as a viable analgesic target, other sodium channels, including Nav1.7 and Nav1.9, are also implicated in nociceptive transmission and may yield additional pharmacologic candidates[13]. The clinical success of Suzetrigine thus not only offers a therapeutic alternative for moderate-to-severe pain but also catalyzes a broader rethinking of pain pharmacotherapy rooted in targeted, non-opioid interventions.
In conclusion, Suzetrigine (VX-548) offers a new and promising approach to acute pain management. Its pharmacologic precision, lack of central nervous system penetration, and absence of addictive potential position as a critical addition to the armamentarium against the global opioid crisis. While its standalone effectiveness is still under investigation, preliminary findings suggest considerable value in multimodal contexts. As clinical practice continues to evolve toward safer, more personalized strategies, Suzetrigine embodies the principles of modern analgesia: efficacy, safety, and commitment to minimizing harm.
-
References
1. Volkow ND, McLellan AT. Opioid Abuse in Chronic Pain—Misconceptions and Mitigation Strategies. N Engl J Med. 2016 Mar;374(13):1253–63. https://doi.org/10.1056/NEJMra1507771 PMID:27028915
2. Keam SJ. Suzetrigine: first Approval. Drugs. 2025 Jun;85(6):845–51. https://doi.org/10.1007/s40265-025-02178-w PMID:40323340
3. Osteen JD, Immani S, Tapley TL, Indersmitten T, Hurst NW, Healey T, et al. Pharmacology and Mechanism of Action of Suzetrigine, a Potent and Selective NaV1.8 Pain Signal Inhibitor for the Treatment of Moderate to Severe Pain. Pain Ther. 2025 Apr;14(2):655–74. https://doi.org/10.1007/s40122-024-00697-0 PMID:39775738
4. Vertex Pharmaceuticals. Suzetrigine (Journavx). Prescribing Information; 2025.
5. RxList. Journavx (Suzetrigine). Pharmacokinetics; 2025.
6. Beninger P. Journavx (suzetrigine). Clin Ther. 2025 May;47(5):400–1. https://doi.org/10.1016/j.clinthera.2025.02.008 PMID:40090791
7. Rind DM, McQueen B, Nikitin D, Lee W, DiStefano MJ, Zemplenyi A, et al. Suzetrigine for Acute Pain; Final Report. Institute for Clinical and Economic Review, March 31, 2025.
8. Hang Kong AY, Tan HS, Habib AS. VX-548 in the treatment of acute pain. Pain Manag. 2024 Sep;14(9):477–86. https://doi.org/10.1080/17581869.2024.2421749 PMID:39552600
9. Bertoch T, D’Aunno D, McCoun J, Solanki D, Taber L, Urban J, et al. Suzetrigine, a Non-Opioid NaV1.8 Inhibitor for Treatment of Moderate-to-Severe Acute Pain: Two Phase 3 Randomized Clinical Trials. Anesthesiology. 2025. https://doi.org/10.1097/ALN.0000000000005460.
10. McCoun J, Winkle P, Solanki D, Urban J, Bertoch T, Oswald J, et al.; VX-548-107 Study Team. Suzetrigine, a Non-Opioid NaV1.8 Inhibitor With Broad Applicability for Moderate-to-Severe Acute Pain: A Phase 3 Single-Arm Study for Surgical or Non-Surgical Acute Pain. J Pain Res. 2025 Mar;18:1569–76. https://doi.org/10.2147/JPR.S509144 PMID:40165940
11. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993 Nov;77(5):1048–56. https://doi.org/10.1213/00000539-199311000-00030 PMID:8105724
12. Volkow ND, Blanco C. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry. 2021 Jan;26(1):218–33. https://doi.org/10.1038/s41380-020-0661-4 PMID:32020048
13. Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nat Rev Neurosci. 2013 Jan;14(1):49–62. https://doi.org/10.1038/nrn3404 PMID:23232607

 ORCID
ORCID