Recibido: 10-03-2022
Aceptado: 18-04-2022
©2022 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 51 Núm. 5 pp. 598-601|https://doi.org/10.25237/revchilanestv5106071204
PDF|ePub|RIS
Abstract
Phantom limb syndrome (PLS) is a condition that occurs in amputee patients that has a wide array of different treatment approaches. We present the case of a patient diagnosed with complex regional pain syndrome (CRPS) of the right upper limb secondary to polytrauma with complete brachial plexus injury, who, after being subjected to multiple successful therapies, was finally taken to amputation. Later, he developed a painful PLS that was difficult to manage, which was treated with a stellate ganglion block (SGB), achieving a significant decrease in pain. This article aims to present a case in which a SGB was used as an adjunct to control acute postoperative PLS in a patient with previous sensitization due to CRPS. The SGB, in this case, performed with local anesthetic plus corticosteroid, constitutes a useful therapeutic alternative for intense postoperative pain in PLS, achieving adequate pain relief without adverse effects. However, its routine use as in acute postoperative pain still lacks sufficient evidence for complete support, therefore we urge the scientific community to undertake more in-depth research concerning this topic in order to create clear guidelines and recommendations.
Resumen
El síndrome del miembro fantasma (PLS, por sus siglas en inglés) es una condición que se presenta en pacientes amputados con un campo extenso para los diferentes enfoques de tratamiento. Presentamos el caso de un paciente diagnosticado con síndrome complejo de dolor regional (SCDR) del miembro superior derecho secundario a politraumatismo con lesión completa del plexo braquial, quien, tras ser sometido a múltiples tratamientos exitosos, fue finalmente llevado a amputación. Posteriormente, desarrolló un PLS doloroso de difícil manejo, el cual fue tratado con un bloqueo del ganglio estrellado (BGE), logrando una disminución significativa del dolor. Este artículo tiene como objetivo presentar un caso en el que se utilizó un BGE como coadyuvante para el control del PLS en el posoperatorio de manera aguda en un paciente con sensibilización previa por SCDR. EL BGE, en este caso, realizado con anestésico local más corticosteroide, constituye una alternativa terapéutica útil para el dolor posoperatorio intenso en PLS, consiguiendo un adecuado alivio del dolor sin efectos adversos. Sin embargo, su uso rutinario como en el dolor postoperatorio agudo, aún carece de evidencia suficiente para un respaldo completo, por lo que invitamos a la comunidad científica a realizar una investigación más profunda sobre este tema para crear pautas y recomendaciones claras.
-
Introduction
The treatment of PLS has pharmacological, interventional, behavioral, and surgical components that one must take into account. Regarding pharmacological therapy, non- opioid analgesics, tricyclic antidepressants or dual inhibitors, neuroleptics, anticonvulsants, opioids, neuromuscular block- ers, ketamine, and capsaicin are frequently used[1]. However, within this article we would like to focus on describing the use of SGB as an adjunct to treat phantom limb pain in the immedi- ate acute postoperative period. The stellate ganglion is formed by the fusion of the inferior cervical and first thoracic sympa- thetic ganglion tracts, located anterior to the transverse process of C7, superior to the first rib and inferior to the subclavian artery[2]. The application of local anesthetic can potentially reduce pain that has a sympathetic component and has proven to be an effective therapy in pathologies that affect the upper extremities, face, and head[2]. The role of the SGB is well de- fined in patients with chronic pain, as it disrupts sympathetic tone, prevents central sensitization, and helps restore normal somatic sensation[3]. Additionally, it has been widely used in upper limb surgeries because it does not produce motor or sen- sory blockade, which allows the surgeon to evaluate the motor and sensory function of the limb in the immediate postopera- tive period[4]; however, its use as an acute postoperative pain therapy is still under debate.
-
Clinical Case
A 40-year-old male patient with a history of polytrauma secondary to a vehicular accident, causing a left humeral fracture with complete brachial plexus injury which required exploration, neurolysis, neurotization of the spinal accessory to suprascapular nerve, and left brachial plexus reconstruction with contralateral C7 transfer. Subsequently, a right to left brachial plexus nerve transfer was performed.
The patient mentioned 1 year of burning and stinging sensations, paresthesias, and painful tingling in the left hand that was exacerbated by mobilization and improved by hand traction and administration of pregabalin and methadone. The VAS was 10/10, with a minimum of 7/10, associated with suicidal thoughts, despair, and total inability to carry out daily activities, with a Lattinen index of 20/20. The patient had antecedents of depression and insomnia, as well as an allergy to morphine. On physical examination of the upper right limb, the strength was found to be 1/5, with generalized hypoesthesia, and allodynia in the back and palm of the hand, with an electromyography that reports severe left brachial plexus injury. It was considered that the patient was presenting with a CRPS of the left upper limb, for which neuromodulation was started with oral gabapentin (600 mg every 6 hours), amitriptyline (50 mg at night), and tramadol (50 mg every 12 hours). No improvement in symptoms was seen in the aforementioned approach, there- fore a fluoroscopy-guided left SGB was indicated with 0.125% bupivacaine, 8 mg of dexamethasone and saline solution, combined for 10 mL of volume (Figure 1).
A decrease in pain was obtained with a VAS of 6/10 during the first day after the procedure; however, the presence of severe pain associated with suicidal thoughts returned, for which the patient was programmed for fluoroscopy guided neurolysis by thermal radiofrequency at 80° C for 90 seconds of the ganglions of the T2-T3 sympathetic chain, which lead to complete analgesia 24 hours post-procedure. Unfortunately, the symptoms reappeared after 48 hours with the same characteristics and intensity, with a VAS of 10/10.
Given the torpid evolution and severe functional limitation of the limb, the patient was taken to a medical board decision where limb disarticulation was decided, to which the patient accepted. The procedure was performed under general anesthesia, with ketamine (50 mg IV bolus), remifentanil (0.15 mcg/ kg/min infusion), dexmedetomidine 0.8 mcg/kg/hr, and lidocaine 1 mg/kg/h, plus an interscalene blockade and transitional analgesia with diclofenac and paracetamol.
In the immediate postoperative period, multimodal management was started with oral neuromodulators (amitriptyline 25 milligrams at night, and pregabalin 300 milligrams every 12 hours), a short cycle of NSAIDs and a hydromorphone (0.4 mg IV bolus) as a rescue therapy. However there was an VAS of 8/10 and perception of a painful phantom limb with poor pain control, therefore venlafaxine, a lidocaine infusion (70 milligrams per hour) and ultrasound-guided SGB were added to the management. The SGB was performed by applying a mixture of 8 milligrams of dexamethasone in 2 mL, 4 mL of 0.5% bupivacaine and 4 mL of 1% lidocaine for a total volume of 10 mL. This procedure was performed on 2 occasions, achieving a VAS of 0/10, alleviation of the painful sensation of the phantom limb and eventual discharge from the hospital.
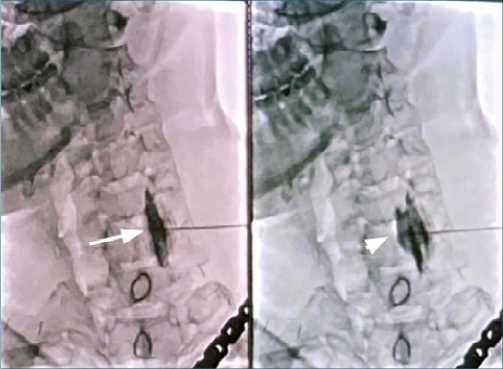
Figure 1. Left Stellate Ganglion Block via Fluoroscopy. Fluoroscopy- guided left cervicothoracic sympathetic block. White Arrow: Location of the longus colli muscle, White Arrowhead: Post-administration of analgesic solution with dispersion of contrast medium.
-
Discussion
This constitutes an interesting case considering the fact that the approach to acute post-operative pain in a patient with previous central and peripheral sensitization, secondary to CRPS of the limb, requires multiple strategies in order to achieve ad- equate analgesia[5]. The anesthesia used for the amputation was a mixed technique of general anesthesia and a brachial
plexus block via the interscalene route. It was considered that the implementation of a continuous regional block through nerve sheath catheters has been shown to provide postoperative analgesia after amputation, however it does not prevent the phantom limb pain seen in this case (Level I evidence). On the other hand, the administration of morphine, gabapentin, ketamine and dextromethorphan reduce the pain of the phan- tom limb (Evidence Level I)[6]; however, in this patient, the allergy to morphine was a significant limiting factor. Regarding the use of systemic lidocaine, there are studies that suggest that it is ineffective; however, it has been shown that contralateral myofascial injection in the painful point equivalent to that of the amputated limb with 0.125% bupivacaine reduces phantom limb pain[6]. In our case, the decision to perform a sympathetic nerve block was based on the failure of drug therapy, the presence of uncontrolled pain in the amputation stump, and the certainty of the involvement of the sympathetic nervous system in the pain mechanism of this patient. Regarding the interventional management in this condition, it is worth recognizing that the most effective interventions are upper limb sympathetic blocks, spinal stimulation and pulsed radiofrequency.
The use of sympathectomy in sustained sympathetic neuropathic pain has shown, in some studies, a significant improvement of pain and quality of life. In a retrospective study that included 105 patients with CRPS and neuropathic pain syndromes of the upper limb, an ultrasound-guided SGB was utilized and was shown to result in significant improvement in the pain scales[7]. However, the use of thoracic paravertebral sympathetic chain neurolysis at T2 and T3 levels provide an alternative with a superior safety profile in terms of complications and greater efficacy, given the contribution of sympathetic innervation of these structures to the upper limbs. A case series from 2013 of 4 male patients suffering from CRPS secondary to brachial plexus injury showed immediate pain relief of more than 75% after 6 months and a decrease in the consumption of analgesics (approximately 50%) after radiofrequency at the levels of T2-T3 was undergone[8]. In 1980, Krainick et al.[9], published an article in which they studied a total of 84 patients with post-amputation pain who underwent spinal stimulation, showing favorable results in the reduction of pain of approximately 50%, and a decrease in consumption of long-term pain relievers. Later in 2013, McAuley et al.[10], published a study in which stimulation of the dorsal root was performed by implantation of stimulators in the epidural space via laminectomy un- der general anesthesia. In this study a total of 12 patients were included, of which 11 had a significant immediate decrease in pain with an average decrease of 65%, however, the evidence in actuality is not strong enough to generate a recommendation.
Finally, regarding the use of radiofrequency, it has gained much recognition as a treatment for neuropathic pain, due to its minimal potential for damage. Described in 1975 as continuous radio frequency (CRF) that uses a high-frequency electric current (500 kHz) to generate a controlled increase in tissue temperature (80-90 °C), causing direct damage to the nociceptive pathway, unlike pulsed radio frequency (PRF) which consists of applying the wave in pulses with lower frequencies (300-500 kHz), with active periods of 20 msg and inactive periods of 480 msg allowing a cooling time that theoretically avoids tissue damage[11]. However, the effectiveness of PRF in the dorsal root ganglion (DRG) is limited to case series. Eldabe et al.[12], performed PRF of the DRG in 8 patients who showed a mean reduction in pain of 52% after a follow-up of 14 months, thus improving their quality of life, and reducing the consumption of analgesics and neuromodulators.
-
Conclusion
The benefit of SGB for the management of acute postoperative pain after amputation was a successful rescue intervention in our case, achieving adequate pain control. However, it does not have sufficient evidence to support its routine use in acute postoperative pain, therefore we urge the scientific com- munity to undertake more in-depth research concerning this topic in order to create clear guidelines and recommendations.
-
References
1. Giummarra MJ, Moseley GL. Phantom limb pain and bodily awareness: current concepts and future directions. Curr Opin Anaesthesiol. 2011 Oct;24(5):524–31. https://doi.org/10.1097/ACO.0b013e32834a105f PMID:21772144
2. Annabi EH, Arefieg J, Shiller S. Stellate Ganglion Blockade. In: Pope JE, Deer TR, editors. Treatment of Chronic Pain Conditions: A Comprehensive Handbook. Springer; 2017. pp. 145–6. https://doi.org/10.1007/978-1-4939-6976-0_40.
3. Bantel C, Trapp S. The role of the autonomic nervous system in acute surgical pain processing – what do we know? Anaesthesia. 2011 Jul;66(7):541–4. https://doi.org/10.1111/j.1365-2044.2011.06791.x PMID:21627623
4. Hurley R, Wu C. Acute Postoperative Pain. In: 2010:2757-2781. https://doi.org/10.1016/B978-0-443-06959-8.00087-X.
5. Kent ML, Hsia HJ, Van de Ven TJ, Buchheit TE. Perioperative Pain Management Strategies for Amputation: A Topical Review. Pain Med. 2017 Mar;18(3):504–19. https://doi.org/10.1093/pm/pnw110 PMID:27402960
6. Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2016 Oct;10(10):CD006380. https://doi.org/10.1002/14651858.CD006380.pub3 PMID:27737513
7. Aleanakian R, Chung BY, Feldmann RE Jr, Benrath J. Effectiveness, Safety, and Predictive Potential in Ultrasound-Guided Stellate Ganglion Blockades for the Treatment of Sympathetically Maintained Pain. Pain Pract. 2020 Jul;20(6):626–38. https://doi.org/10.1111/papr.12892 PMID:32255250
8. Chen CK, Phui VE, Nizar AJ, Yeo SN. Percutaneous t2 and t3 radiofrequency sympathectomy for complex regional pain syndrome secondary to brachial plexus injury: a case series. Korean J Pain. 2013 Oct;26(4):401–5. https://doi.org/10.3344/kjp.2013.26.4.401 PMID:24156009
9. Krainick JU, Thoden U, Riechert T. Pain reduction in amputees by long-term spinal cord stimulation. Long-term follow-up study over 5 years. J Neurosurg. 1980 Mar;52(3):346–50. https://doi.org/10.3171/jns.1980.52.3.0346 PMID:6153710
10. McAuley J, van Gröningen R, Green C. Spinal cord stimulation for intractable pain following limb amputation. Neuromodulation. 2013 Nov-Dec;16(6):530–6. https://doi.org/10.1111/j.1525-1403.2012.00513.x PMID:23009132
11. Martín-Arroyo T. M J. Radiofrecuencia pulsada: pasan los años y seguimos con las mismas incógnitas. Rev Soc Esp Dolor. 2016;23(4):167–9. https://doi.org/10.20986/resed.2016.3473/2016.
12. Dorsal Root Ganglion (DRG) Stimulation in the Treatment of Phantom Limb Pain. (PLP) – Eldabe – 2015 – Neuromodulation: Technology at the Neural Interface – Wiley Online Library. Accessed March 29, 2021. https://onlinelibrary.wiley.com/doi/abs/10.1111/ner.12338

 ORCID
ORCID
 Creative Commons Attribution
Creative Commons Attribution