Rasha Hamed 1 ,*, Saeid Elsawy 1
Recibido: 11-05-2021
Aceptado: 21-10-2021
©2022 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 51 Núm. 2 pp. 184-190|https://doi.org/10.25237/revchilanestv5107021620
PDF|ePub|RIS
Omalgia luego de quistectomía ovárica laparoscópica, efecto de fentanyl comparado con dexametasona
Abstract
Background: General anesthesia (GA) was the usual anesthetic technique used for laparoscopic interventions, however regional anesthesia in the laparoscopic field started to gain familiarity. Shoulder pain is a major intraoperative problem that might hinder facilitation of laparoscopic interventions under spinal anesthesia. Aim of the study: Evaluate the effect of intrathecal addition of dexamethasone versus fentanyl on incidence and severity of intraoperative shoulder tip pain during gynecological laparoscopic asurgeries. Methods: 120 patients, were randomly assigned into three groups. Group D: 40 patients received 15 mg bupivacaine and 8 mg dexamethasone intrathecally. Group F: 40 patients received 15 mg bupivacaine and 25 pg fentanyle intrathecally. Group C: 40 patients received 15 mg bupivacaine and normal saline intrathecally. Results: Number of patients who experienced intraoperative shoulder pain was significantly lower in Group F (17) and Group D (19) than Group C (31); P = 0.008. with no ststistical difference detected between Group D and C (p value 1). Only 2 patients in Group D and F suffered moderate pain intensity in comparison to 9 patients in Group C; P =0.02. Incidence of postspinal shivering was lower in Group D and F in comparison to Group C; P 0.02. Conclusión: Intrathecal dexamethasone is as effective as intrathecal fentanyle in reducing incidence and severity of shoulder tip pain during laparoscopic ovarian cystectomy under spinal anesthesia.
Resumen
Introducción: La anestesia general (AG) era la técnica anestésica habitual utilizada para las intervenciones laparoscópicas, sin embargo, la anestesia regional en el campo laparoscópico comenzó a ganar familiaridad. El dolor de hombro es un problema intraoperatorio importante que podría dificultar la facilitación de las intervenciones laparoscópicas bajo anestesia espinal. Objetivo del estudio: Evaluar el efecto de la adición intratecal de dexametasona versus fentanilo sobre la incidencia y severidad del dolor intraoperatorio en la punta del hombro durante cirugías laparoscópicas ginecológicas. Métodos: 120 pacientes, fueron asignados aleatoriamente en tres grupos. Grupo D: 40 pacientes recibieron 15 mg de bupivacaína y 8 mg de dexametasona por vía intratecal. Grupo F: 40 pacientes recibieron 15 mg de bupivacaína y 25 pg de fentanilo por vía intratecal. Grupo C: 40 pacientes recibieron 15 mg de bupivacaína y solución salina normal por vía intratecal. Resultados: El número de pacientes que experimentaron dolor de hombro intraoperatorio fue significativamente menor en el Grupo F (17) y el Grupo D (19) que en el Grupo C (31); P = 0,008. sin diferencia estadística detectada entre el Grupo D y C (valor de p 1). Solo 2 pacientes del Grupo D y F sufrieron dolor de intensidad moderada en comparación con 9 pacientes del Grupo C; P = 0,02. La incidencia de escalofríos posespinales fue menor en el Grupo D y F en comparación con el Grupo C; P 0,02. Conclusión: La dexametasona intratecal es tan eficaz como el fentanilo intratecal para reducir la incidencia y la gravedad del dolor en la punta del hombro durante la cistectomía ovárica laparoscópica bajo anestesia espinal.
Introduction of laparoscope as a surgical tool modulated the medical field, enhanced postoperative recovery and reduced hospital stay, hence minimized the medical cost[1]. General anaesthesia (GA) traditionally was believed to be the only suitable anaesthetic technique for laparoscopic procedures, because pneumoperitoneum is expected to induce respiratory and cardiovascular compromise which thought to be better regulated by GA[2].
Recently, laparoscopic procedures under RA started to gain practical acceptance due to its appreciatable merits over GA, where there is no manipulation of the airway, lower aspiration risk , reduced medical cost, and reduced PONV (postoperative nausea and vomiting)[3]. Laparoscopy attributed shoulder tip pain (STP) could be the major intraoperative complaint that limits RA practice in laparoscopic interventions[4]. Various trials were carried out to minimize laparoscopic induced shoulder tip pain[4],[5],[6],[7], that has a recorded postoperative incidence of 35% to 80%[8]. Intrathecal dexamethasone has been used to improve quality of spinal anesthesia without serious side effects recorded[9],[10],[11]. Dexamethasone is thought to exert its analgesic activity through inhibiting inflammation and preventing C fiber mediated nociception and blocking ectopic neuronal firing[11]. Fentanyl is a lipophilic opioid famously used intrathecally with a relatively approved intraoperative antinociceptive effects[12],[13],[14]. Aim of this study was evaluating the effect intrathecal dexamethasone versus intrathecal fentanyl on incidence and severity of shoulder tip pain during gynecological laparoscopic procedures.
Trial registration and Ethical consideration: This prospective, double-blind, randomized trial was done in Assiut university hospital after local ethical committee approval (IRB 13700331). The study was registered on clinicaltrials.gov (NCT04779060).
The methodology followed in this trial was in compliant with the Helsinki Declaration of 1975, revised in 2013. An in- formed written consent was obtained from the patients at the preoperative evaluation after explaining the protocol of the study, possible benefits, risks, and alternative managements.
Inclusion criteria: Elective surgery, age 20-40 years, and ASA 1 or 11.
Exclusion Criteria: Contraindications to regional anaesthesia, hepatic or renal diseases, pregnancy and BMI >30.
Primary outcome: – incidence and severity of intraoperative shoulder tip pain.
Secondary outcome: – perioperative shivering and PONV.
Randomization: patients randomly assigned into one of 3 groups by random allocation software. Numbers are kept in colsed envelopes, 30 minutes before operation the patient randomly choose an envelope from a mixing bowl and handle it to the anesthesia assistant who was responsible of local anesthetic mixture preparation, but not involved in block handling or data gathering. Participants, surgeon, and anaesthetist responsible for block handling and data collection remained blind to the study groups allocation.
Study Groups: 120 patients were randomly assigned into; Group D (n = 40): – received intrathecal 0.5% hyperbaric Bupivacaine 3 ml (15 mg) plus 8 mg dexamethasone, Group F (n = 40): – received intrathecal hyperbaric Bupivacaine 3 ml (15 mg) plus 25 pg fentanyl and Group C (n = 40): – received intrathecal hyperbaric Bupivacaine 3 ml (15 mg) plus 2 ml normal saline.
Anaesthesia technique: patients were premedicated with IV ondansetron 4 mg and preloaded with 10 ml/kg normal saline 30 minutes before surgery. At the operating theatre standard monitoring with NIBP, 5 leads ECG, capnography, and pulse oximetry were attached to the patient and baseline data were re- corded. Spinal anesthesia was given in complete a septic technique and in sitting position at L3-4, L4-5 intervertebral space, 25 gauge Quincke’s spinal needle was introduced through mid- line approach. After intrathecal drug administration patients were placed in supine position, assessment of sensory block was done every 2 min using pinprick technique, the operating table was handled untill T6 level was approached. Systolic blood pressure < 90 was considered hypotension and treated with IV ephedrine and fluid bolus. HR < 55 WAS considered bradycardia and treated with IV atropine 0.05 mg/kg.
Assessments of intraoperative shoulder pain was done by VAS score 0 = no pain and 10 = most severe pain; VAS score of 1,2, 3 is mild pain, VAS score of 4,5,6 is moderate pain and VAS of 7,8,9,10 is severe pain. Intraoperative pain was treated with 25 mg ketamine repeated as needed if pain did not relieve within 3 minutes after injection. General anesthesia was ready upon patient request or if pain intolerable. Shivering intensity was evaluated by the validated Crossly and Mahajan scale, where “0 = No shivering, 1 = piloerection or peripheral vasoconstriction but no visible shivering, 2 = muscular activity in only one muscle group, 3 = muscular activity in more than one muscle group, and 4 = whole body shivering”. Shivering was treated with 25 mg mepridine repeated if shivering reappeared.
Sample size calculation: The sample size calculated using G*Power version 3.1 based on a previous study[15], where mean VAS in dexamethasone group was 3.88 ±1. This sample size was estimated to be able to detect a 20% difference in mean VAS score between both groups, with a = 0.05 35 patients of each group would yield 90% power. We enrolled 40 in each group to compensate for dropouts.
Statistical analysis: Data analysis was done by the IBM SPSS 20.0 software. Normality tested by Kolmogorov-Smirnov test. Categorical variables were represented as number and percent (N, %), while continuous variables were described by mean and standard deviation (Mean, SD). Analysis of categorical data was done by Chi-square test, while continuous variables were analyzed by ANOVA for parametric data and Kruskal-Walis test for Nonparametric data. A two tailed p value < 0.05 was considered statistically significant.
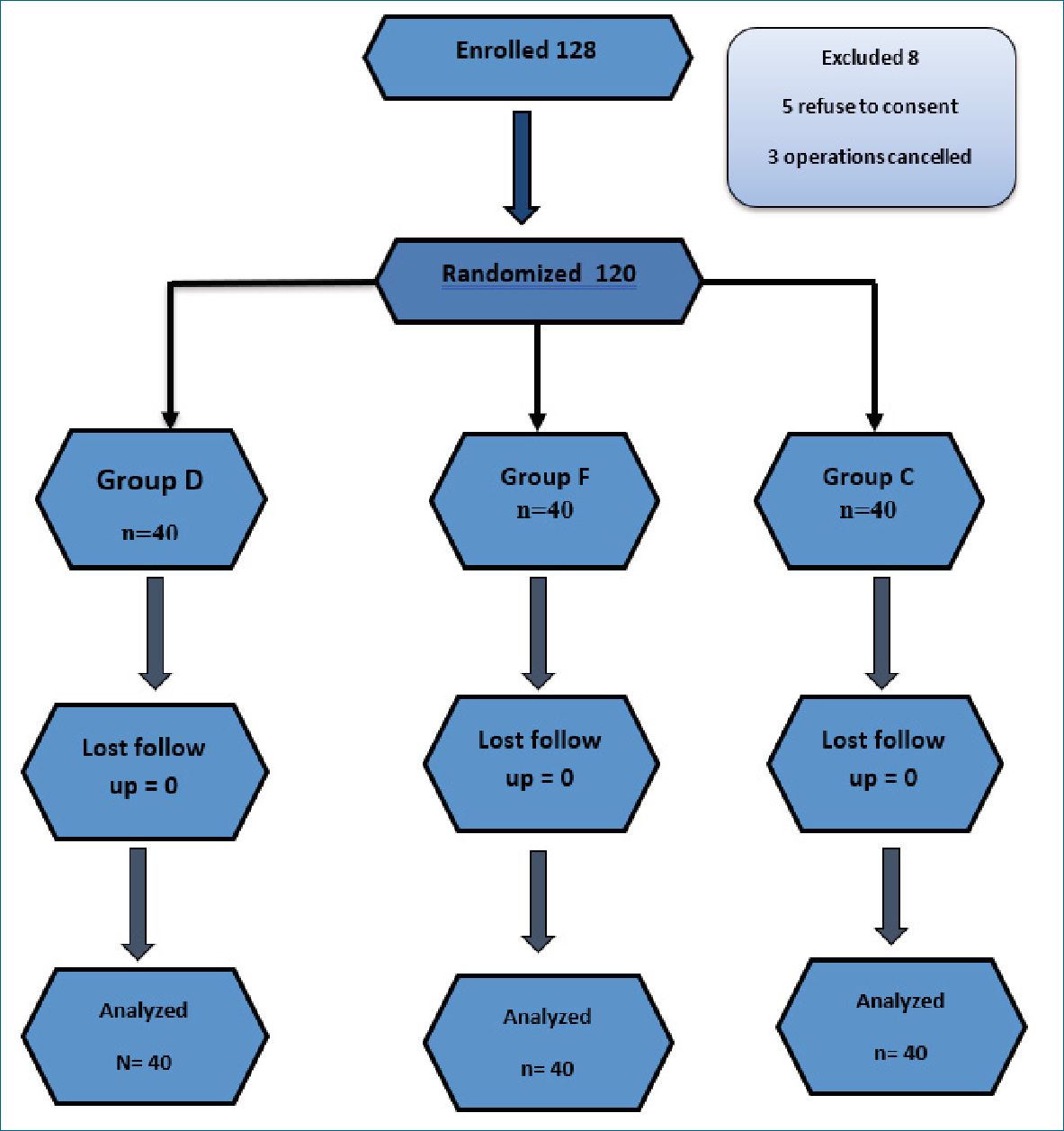
Figure 1. Flow chart.
| Table 1. Demographic data and surgical characteristics | ||||
| Group D | Group F | Group C | P-Value | |
| n = 40 | n = 40 | n = 40 | ||
| Age | 28.3 ± 6 | 29.6 ± 5 | 27.6 ± 5 | 0.25 |
| BMI | 27 ± 2.4 | 26 ± 3 | 25 ± 2 | 0.35 |
| Surgery duration | 49 ± 10 | 47 ± 9 | 46 ± 8 | 0.5 |
| Intraoperative VAS score | mean 1.2 ± 1.4 | 0.9 ± 1.3 | 2.1 ± 1.7 | 0.002** |
| Post P1 | range (1-5) | (1-4) | (1-5) | 0.5 |
| hoc P2 | 0.007** | |||
| P3 | 0.001** | |||
| Ketamine dose | 16 ± 27 | 14 ± 26 | 35 ± 41 | 0.02* |
| Post P1 | 0.5 | |||
| hoc P2 | 0.04* | |||
| P3 | 0.008** | |||
| PONV | 3/40 | 17/40 | 12/40 | 0.002** |
| Post P1 | < 0.001 | |||
| hoc P2 | 0.02 | |||
| P3 | 0.2 | |||
Mean ± SD; ANOVA for parameteric variables. Kruskal-wallis test for non-parametric variables; P < 0.05 is significant, P1= comparison between group D and F; P 2 = comparison between group D and C; P3 = comparison between group F and C.
120 patients completed the study with 40 patients in each group (Figure1). No statistical difference was found in demographic data (age, BMI) or surgery duration between both groups (Table 1). Mean arterial blood pressure was comparable between the study groups till the end of the operation, mean- while heart rate was significantly lower in Group F than Group D and Group C during operation (Figure 2). Incidence of intraoperative shoulder pain was significantly higher in Group C (77.5%) in comparison to Group D (47.5%) and F (42.5%); P Value = 0.006, with mean VAS score (1.2 ± 1.4 vs 0.9 ± 1.3 vs 2.1 ± 1.7; P 0.001) in Group D, F and Group C respectively (Table 2 and 3). However, no statistical difference in incidence of intraoperative shoulder pain between Group D and Group F; P value = 1, and the Severity of the reported shoulder pain was comparable between both groups; for mild pain 17 patients in Group D vs 15 patients in Group F with P = 1. Two patients in Chi-square test; P <0.05 is significant; P value = comparison between the three groups; P1 = comparison between group D and F; P2 = comparison between group D and C; P3 = comparison between group F and C; Group D is dexamethasone; Group F is fentanyl; Group C is control group.
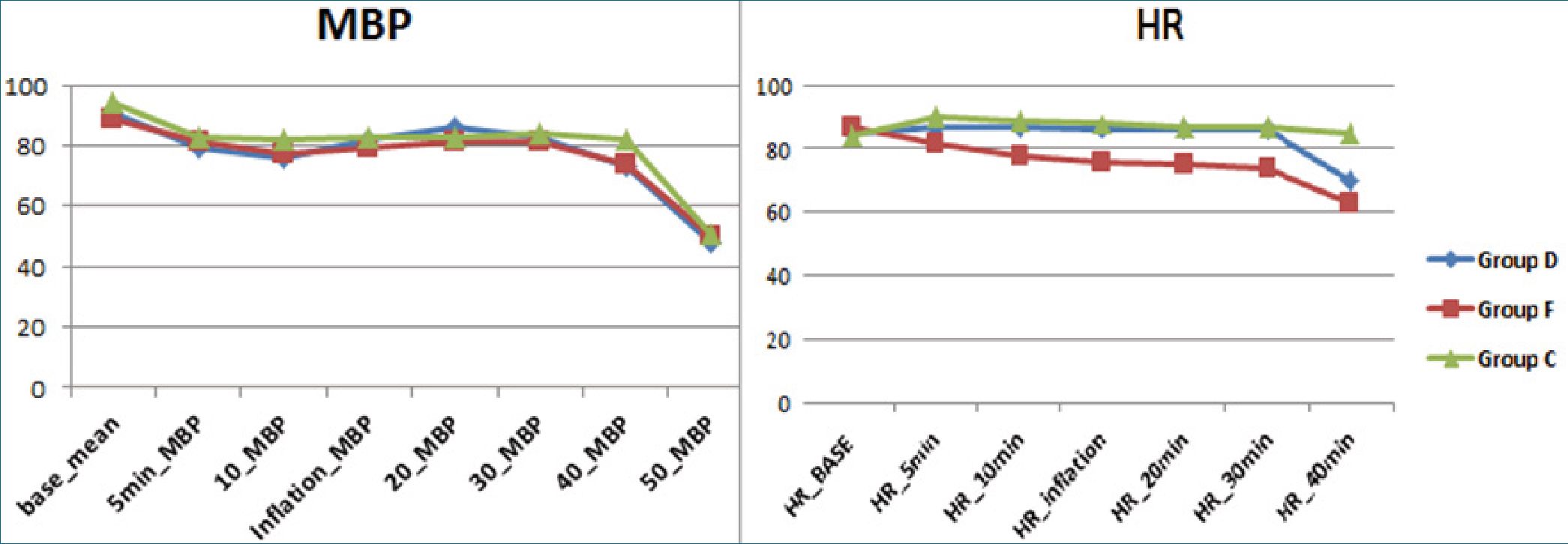
Figure 2. Intraoperative hemodynamics.
Table 2. Intraoperative shoulder pain Intraoperative shoulder pain
| No pain | Mild pain (VAS 1-3) | Moderate pain (VAS 4-6) | Severe pain (VAS 7-10) | Incidence of shoulder pain | Percent of Pain reduction | |
| Group D (n = 40) | 21/40 | 17/40 | 2/40 | 0/40 | 47.5% | 35.5% |
| Group F (n = 40) | 23/40 | 15/40 | 2/40 | 0/40 | 42.5% | 45% |
| Group C (n = 40) | 9/40 | 22/40 | 9/40 | 0/40 | 77.5% | |
| P value | 0.006** | 0.3 | 0.02* | 1 | ||
| P 1 | 1 | 1 | 1 | |||
| P2 | 0.03* | 0.8 | 0.03* | |||
| P3 | 0.01* | 0.4 | 0.03* |
| Table 3. Shivering grades | |||||
| No shivering | Shivering grades
Shivering (grade 1) Shivering (grade 2) |
Shivering (grade 3) | Shivering grade 4 | ||
| Group D (n = 40) | 24/40 | 10/40 | 4/40 | 2/40 | 0/40 |
| Group F (n = 40) | (60%) 16/40 | 13/40 | 7/40 | 3/40 | 1/40 |
| Group C n = 40 | (40%) 12/40 | 8/40 | 10/40 | 7/40 | 3/40 |
| P value | (30%)
0.02* |
0.4 | 0.1 | 0.14 | 0.16 |
Chi-square test; P < 0.05 is significant.
each group complaint from moderate pain severity, no patients reported sever pain in both groups (Table 2, Figure 3). Mean amount of ketamine used intraoperative was lower in group D (16 ± 27) and Group F (14 ± 26) in comparison to Group C (34 ± 41); P = 0.01 (Table 1). Incidence of shivering was lower in Group D (40%) and Group F (60%) than Group C(70%); P 0.02. However, NO difference detected when comparing the grades of shivering between the three groups (Table 3 and
Figure 4). For PONV; high statistical significant difference was found between the three groups (3 vs 17 vs 12; P < 0.002 for Group D, Group F and Group C respectively) (Table 1).
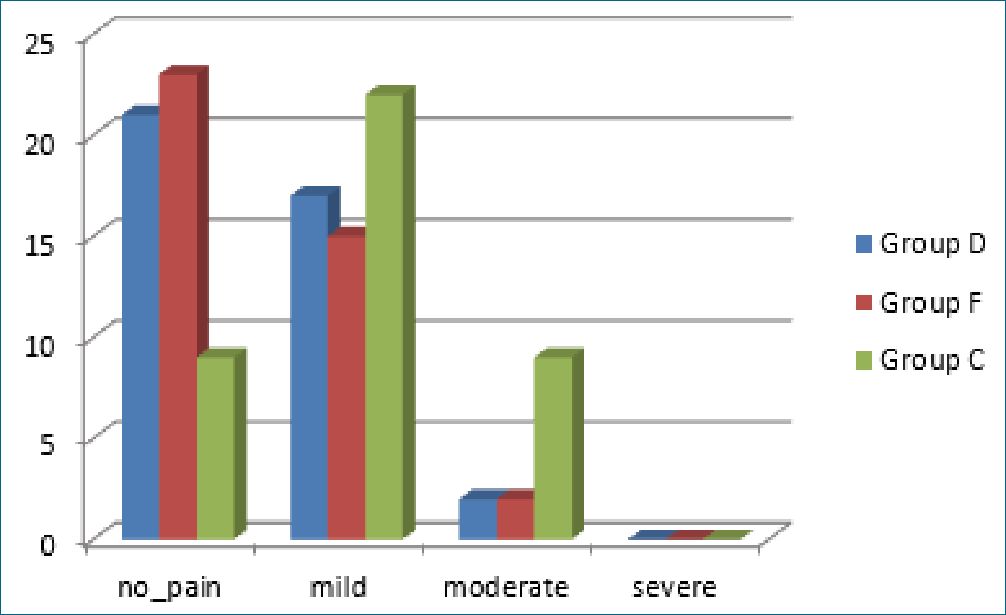
Figure 3. Pain grades between the study groups.
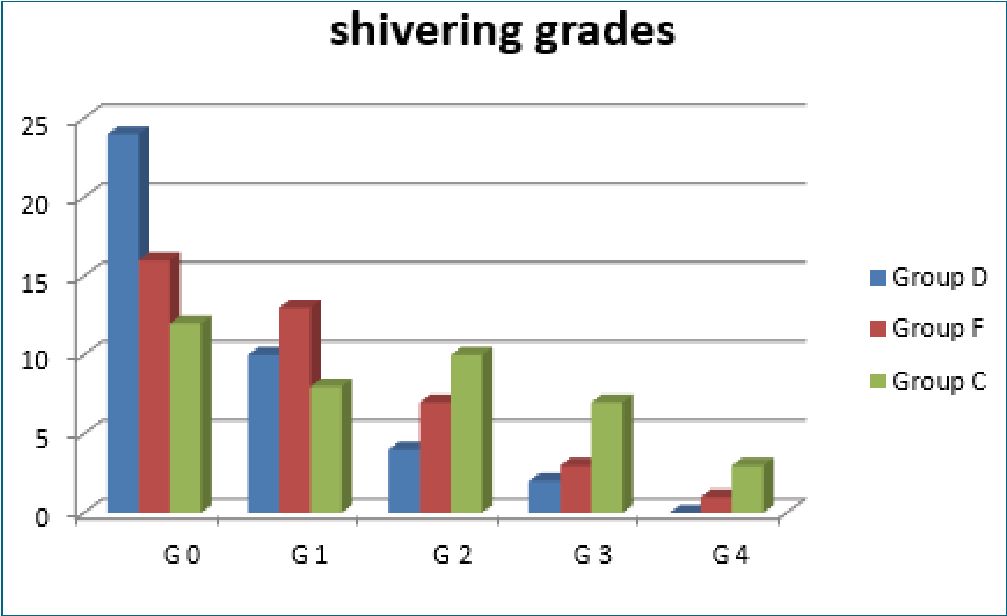
Figure 4. Shivering grades.
General anesthesia generally was preferred for laparoscopic intervetntion, however spinal anesthesia provides a good choice for laparoscopic interventions; due to intense muscle relaxation, uneventual anesthesia recovery, less incidence of PONV and less hospital stay. Different types of laparoscopic procedures were reported to be successfully done under spinal anesthesia[16],[17],[18],[19]. The two main concerns of laparoscopic procedures under spinal anesthesia are; pneumoperitoneum induced respiratory compromise and shoulder pain. However, spontaneous breathing is better than intubation and mechanical ventilation disrupted physiology, which requires 24 h to return to normal preintubation condition[20].
This study evaluated the effect of intrathecal dexamethasone versus fentanyl on laparoscopic induced shoulder pain incidence and severity under spinal anesthesia. Dexamethasone was nearly as effective fentanyl in reducing incidence and severity of shoulder pain and facilitating ovarian cystectomy under spinal anesthesia. Antinociceptive effects of dexamethasone could be explained by its neuroantiinflammatory effects and its ability to prevent C fiber mediated nociception and blocking ectopic neuronal firing[11]. Furthermore, analgesic effects of intrathecal dexamethasone might be related to its ability to regulate the nuclear factor-kB at the spinal cord which is responsible for “central sensitization” inhibition[21]. More- over, dexamethasone inhibits peripheral as well as central prostaglandin synthesis at the level of the spinal cord, minimizing the sensitization of both the inflammatory and the pain path- ways[22]. Intrathecal use of both dexamethasone and fentanyle remains off label, however previous trials demonstrated that no serious complications were recorded from the intrathecal use of both drug [9],[23],[24],[25],[26],[27].
Dexamethasone is a water soluble nonparticulated drug that has been used intrathecaly since 1950s in neoplastic meningitis and radicular pain[28]. Animal studies demonstrated that continuous intrathecal use of dexamethasone (12.5 ng/h) is free from neurotoxicity adverse effects[28]. Furthermore, dexamethasone had demonstrated a cytoprotective effects against bupivacaine and lidocaine induced neurotoxicity by dual mechanisms; first, it stabilizes the mitochondrial transmembrane potential of the neuronal cells preventing local anesthetics induced neuronal cell rupture, LDH leakage and nuclear condensation[29].
Secondly, dexamethasone increases the phosphorylation of Akt (a critical kinase that promotes neuronal cell viability)[29], [30].
Despite the ability of local anesthetics adjuvants used in our study to attenuate intraoperative shoulder tip pain, incidence in treatment groups still high, which indicates that spinal anesthesia could not be a standard technique for all patients and all types of laparoscopic interventions. Other factors that shared in facilitating our study are the use of relatively low insufflation pressure < 13 cmH2O, uncomplicated short surgery duration < 45 min, calm patients who understand the nature of the maneuver and the benefits of regional anesthesia over general anesthesia, actually anxious patient might be a contraindication of spinal anesthesia in such surgical interventions, and skillful well trained surgeon.
In agreement with our results; Sung et al., found that addition of 10 pg fentanyle to bupivacaine resulted in 25% reduction the “incidence of shoulder tip pain” during laparoscopic inguinal hernia repair under spinal anesthesia[31]. Asgari et al., compared the analgesic efficacy of IV fentanyl versus transcutaneous electrical nerve stimulation (TENS) in patients who experienced shoulder pain during gynecological surgery under spinal anesthesia, they reported that TENS was not better than fentanyl in relieving intraoperative shoulder pain[32]. Furthermre, Mohtadi et al., reported that a single dose of preoperative IV dexamethasone significantly reduced postoperative VAS score and mepridine consumption in laparoscopic cholecystectomy patients[15]. Asgari et al., found that intraperitoneal irrigation with 16 mg dexamethasone at the end of laparoscopic gynecological procedures significantly reduced postoperative shoulder pain in comparison to control group where saline was used to irrigate the surgical field[33]. Shoulder pain after laparoscope is multifactorial in nature, the most accepted cause is inflammatory mediators release due to pneumoperitoneum, hence comes the role of dexamethasone as a potent anti-inflammatory in relieving pain[34].
For perioperative anesthesia induced shivering; both study drugs reduced incidence of shivering in comparison to control group, however, intrathecal dexamethasone was comparable to fentanyle in reducing incidence and severity of perioperative shivering. This finding is in agreement with previous studies; Moeen and Moeen compared the antishvering effect of intra- thecal dexamethasone and mepridine, they concluded that efficacy of intrathecal dexamethasone in reducing shvering was highly comparable to intrathecal mepridine[26]. Ismaiel et al., compared antishivering effect of dexamethasone with dexmedetomidine in patients undergoing cesarean section, they concluded that both drugs were comparable in their efficacy in reducing post-spinal shivering[35]. Many studies evaluated the antishevering effect of IV Dexamethasone[36],[37],[38],[39], yet, its intrathecal efficacy in post spinl shivering needs fur- ther evaluation. Incidence of PONV was lower in dexamethasone group than fentanyle and control group. Efficacy of IV dexamethasone in PONV is well recorded in many studies[40]. However, its intrathecal effect on PONV not yet evaluated. The perioperative antiemetic mechanisms of dexamethasone not fully understood; depletion of GABA Stores, inhibition of central synthesis and release of prostaglandens and serotonin and decrease of the BBB permeability to emesis induced toxins are the postulated antiemetic mechanisms of dexamethasone[40]. Limitation of the current study did not measure serum level of the study medications to confirm or declare the role of systemic absorption of these adjuvants as a possible mechanism of analgesia. Our primary outcome was intraoperative shoulder pain, we did not extend evaluation of shoulder pain to the postoperative period, shoulder pain after laparoscope might extend up to 72 h. This is because of the nature of the laparoscopic gynecological interventions in our institute as a one day surgery. lastly, the relatively small sample size.
Intrathecal dexamethasone is as effective as intrathecal fentanyle in reducing incidence and severity of shoulder tip pain during laparoscopic ovarian cystectomy under spinal anesthesia.
References
1. Bajwa SJ, Kulshrestha A. Anaesthesia for laparoscopic surgery: general vs regional anaesthesia. J Minim Access Surg. 2016 Jan-Mar;12(1):4–9. https://doi.org/10.4103/0972-9941.169952 PMID:26917912
2. Bajwa SJ, Takrouri MS. Innovations, improvisations, challenges and constraints: the untold story of anesthesia in developing nations. Anesth Essays Res. 2014 Jan-Apr;8(1):1–2. https://doi.org/10.4103/0259-1162.128890 PMID:25886094
3. Liu J, Zheng X, Zhang Y. Comparative effects on three anesthesia methods in gynecologic laparoscopic surgery. Int J Clin Exp Med. 2017;10(4):6950–7.
4. Ghodki PS, Sardesai SP, Thombre SK. Evaluation of the effect of intrathecal clonidine to decrease shoulder tip pain in laparoscopy under spinal anaesthesia. Indian J Anaesth. 2010 May;54(3):231–4. https://doi.org/10.4103/0019-5049.65370 PMID:20885870
5. Phinchantra P, Bunyavehchevin S, Suwajanakorn S, Wisawasukmongchol W. The preemptive analgesic effect of celecoxib for day-case diagnostic laparoscopy. J Med Assoc Thai. 2004 Mar;87(3):283–8. PMID:15117045
6. Cunniffe MG, McAnena OJ, Dar MA, Calleary J, Flynn N. A prospective randomized trial of intraoperative bupivacaine irrigation for management of shoulder-tip pain following laparoscopy. Am J Surg. 1998 Sep;176(3):258–61. https://doi.org/10.1016/S0002-9610(98)00150-0 PMID:9776154
7. Narchi P, Benhamou D, Fernandez H. Intraperitoneal local anaesthetic for shoulder pain after day-case laparoscopy. Lancet. 1991 Dec;338(8782-8783):1569–70. https://doi.org/10.1016/0140-6736(91)92384-E PMID:1683981
8. Tsai HW, Chen YJ, Ho CM, Hseu SS, Chao KC, Tsai SK, et al. Maneuvers to decrease laparoscopy-induced shoulder and upper abdominal pain: a randomized controlled study. Arch Surg. 2011 Dec;146(12):1360–6. https://doi.org/10.1001/archsurg.2011.597 PMID:22184293
9. Bani-Hashem N, Hassan-Nasab B, Pour EA, Maleh PA, Nabavi A, Jabbari A. Addition of intrathecal Dexamethasone to Bupivacaine for spinal anesthesia in orthopedic surgery. Saudi J Anaesth. 2011 Oct;5(4):382–6. https://doi.org/10.4103/1658-354X.87267 PMID:22144925
10. El-Shourbagy MA, Mammdouh AM, Shawky ME, Mohamed HA. Addition of intrathecal dexamethasone to bupivacaine for spinal anesthesia in cesarean section. Evidence Based Women’s Health Journal. 2019;9(2):416–24. https://doi.org/10.21608/ebwhj.2019.33474.
11. El-Shourbagy MA, Mammdouh AM, Shawky ME, Mohamed HA. Addition of intrathecal dexamethasone to bupivacaine for spinal anesthesia in cesarean section. Evidence Based Women’s Health Journal. 2019;9(2):416–24. https://doi.org/10.21608/ebwhj.2019.33474.
12. Hamber EA, Viscomi CM. Intrathecal lipophilic opioids as adjuncts to surgical spinal anesthesia. Reg Anesth Pain Med. 1999 May-Jun;24(3):255–63. https://doi.org/10.1097/00115550-199924030-00015 PMID:10338179
13. Weigl W, Bieryło A, Krzemień-Wiczyńska S, Mayzner-Zawadzka E. [Comparative study of postoperative analgesia after intrathecal administration of bupivacaine with fentanyl or morphine for elective Caesarean section]. Anestezjol Intens Ter. 2009 Jan-Mar;41(1):28–32. PMID:19517674
14. Biswas BN, Rudra A, Bose BK, Nath S, Chakrabarthy S. Intrathecal Fentanyl With Hyperbaric Bupivacaine Improves Analgesia During Caesarean Delivery And In Early Post-Operative Period. Indian J Anaesth. 2002;46(6):469.
15. Mohtadi A, Nesioonpour S, Salari A, Akhondzadeh R, Masood Rad B, Aslani SM. The effect of single-dose administration of dexamethasone on postoperative pain in patients undergoing laparoscopic cholecystectomy. Anesth Pain Med. 2014 Aug;4(3):e17872. https://doi.org/10.5812/aapm.17872 PMID:25237639
16. Donmez T, Erdem VM, Uzman S, Yildirim D, Avaroglu H, Ferahman S, et al. Laparoscopic cholecystectomy under spinal-epidural anesthesia vs. general anaesthesia: a prospective randomised study. Ann Surg Treat Res. 2017 Mar;92(3):136–42. https://doi.org/10.4174/astr.2017.92.3.136 PMID:28289667
17. Yu G, Wen Q, Qiu L, Bo L, Yu J. Laparoscopic cholecystectomy under spinal anaesthesia vs. general anaesthesia: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 2015 Dec;15(1):176. https://doi.org/10.1186/s12871-015-0158-x PMID:26634822
18. Li L, Pang Y, Wang Y, Li Q, Meng X. Comparison of spinal anesthesia and general anesthesia in inguinal hernia repair in adult: a systematic review and meta-analysis. BMC Anesthesiol. 2020 Mar;20(1):64. https://doi.org/10.1186/s12871-020-00980-5 PMID:32156258
19. Kaya Uğur B, Pirbudak L, Öztürk E, Balat Ö, Uğur MG. Spinal versus general anesthesia in gynecologic laparoscopy: A prospective, randomized study. Turk J Obstet Gynecol. 2020 Sep;17(3):186–95. https://doi.org/10.4274/tjod.galenos.2020.28928 PMID:33072423
20. Sinha R, Gurwara AK, Gupta SC. Laparoscopic surgery using spinal anesthesia. JSLS. 2008 Apr-Jun;12(2):133–8. PMID:18435884
21. Bastos LF, Vago JP, Caux TR, Costa BL, Godin AM, Menezes RR, et al. Delay of neuropathic pain sensitization after application of dexamethasone-loaded implant in sciatic nerve-injured rats. Braz J Pharm Sci. 2019;55:55. https://doi.org/10.1590/s2175-97902019000218112.
22. Gordon KG, Choi S, Rodseth RN. The role of dexamethasone in peripheral and neuraxial nerve blocks for the management of acute pain. Southern African Journal of Anaesthesia and Analgesia. 2016;22(6):163–9. https://doi.org/10.1080/22201181.2016.1251063.
23. Kotani N, Kushikata T, Hashimoto H, Kimura F, Muraoka M, Yodono M, et al. Intrathecal methylprednisolone for intractable postherpetic neuralgia. N Engl J Med. 2000 Nov;343(21):1514–9. https://doi.org/10.1056/NEJM200011233432102 PMID:11087880
24. Sugita K, Kobayashi S, Yokoo A, Inoue T. Intrathecal steroid therapy for post-traumatic visual disturbance. Neurochirurgia (Stuttg). 1983 Jul;26(4):112–7. https://doi.org/10.1055/s-2008-1053622 PMID:6688660
25. El-Shourbagy MA, Mammdouh AM, Shawky ME, Mohamed HA. Addition of intrathecal dexamethasone to bupivacaine for spinal anesthesia in cesarean section. Evidence Based Women’s Health Journal. 2019;9(2):416–24. https://doi.org/10.21608/ebwhj.2019.33474.
26. Moeen SM, Moeen AM. Intrathecal dexamethasone vs. meperidine for prevention of shivering during transurethral prostatectomy: a randomized controlled trial. Acta Anaesthesiol Scand. 2017 Aug;61(7):749–57. https://doi.org/10.1111/aas.12920 PMID:28626868
27. Uppal V, Retter S, Casey M, Sancheti S, Matheson K, McKeen DM. Efficacy of intrathecal fentanyl for cesarean delivery: a systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Anesth Analg. 2020 Jan;130(1):111–25. https://doi.org/10.1213/ANE.0000000000003975 PMID:30633056
28. Kroin JS, Schaefer RB, Penn RD. Chronic intrathecal administration of dexamethasone sodium phosphate: pharmacokinetics and neurotoxicity in an animal model. Neurosurgery. 2000 Jan;46(1):178–82. https://doi.org/10.1093/neurosurgery/46.1.178 PMID:10626948
29. Ma R, Wang X, Lu C, Li C, Cheng Y, Ding G, et al. Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience. 2010 May;167(2):329–42. https://doi.org/10.1016/j.neuroscience.2009.12.049 PMID:20038443
30. Nair VD, Olanow CW. Differential modulation of Akt/glycogen synthase kinase-3beta pathway regulates apoptotic and cytoprotective signaling responses. J Biol Chem. 2008 May;283(22):15469–78. https://doi.org/10.1074/jbc.M707238200 PMID:18387957
31. Sung TY, Kim MS, Cho CK, Park DH, Kang PS, Lee SE, et al. Clinical effects of intrathecal fentanyl on shoulder tip pain in laparoscopic total extraperitoneal inguinal hernia repair under spinal anaesthesia: a double-blind, prospective, randomized controlled trial. J Int Med Res. 2013 Aug;41(4):1160–70. https://doi.org/10.1177/0300060513490083 PMID:23839277
32. Asgari Z, Tavoli Z, Hosseini R, Nataj M, Tabatabaei F, Dehghanizadeh F, et al. A comparative study between transcutaneous electrical nerve stimulation and fentanyl to relieve shoulder pain during laparoscopic gynecologic surgery under spinal anesthesia: a randomized clinical trail. Pain Res Manag. 2018 Mar;2018:9715142. https://doi.org/10.1155/2018/9715142 PMID:29743962
33. Asgari Z, Mozafar-Jalali S, Faridi-Tazehkand N, Sabet S. Intraperitoneal dexamethasone as a new method for relieving postoperative shoulder pain after gynecologic laparoscopy. Int J Fertil Steril. 2012 Apr;6(1):59–64. PMID:25505513
34. Wang Z, Cao Y, Chang Y. [Shoulder pain after laparoscopic cholecystectomy]. Zhonghua Wai Ke Za Zhi. 2001 Nov;39(11):858–60. PMID:11930742
35. Ismaiel MA, El Safty OM, El-Agamy AE, Mohamed OM, Ali MM. A comparative study between dexmedetomidine and dexamethasone as an intrathecal adjuvant for prevention of perioperative shivering in cesarean section. Ain-Shams Journal of Anesthesiology. 2020;12(1):1–9. https://doi.org/10.1186/s42077-020-00102-w.
36. Khosravi A, Moinvaziri MT, Esmaili MH, Farbood AR, Nik-Khoo H, Yarmohammadi H. Treatment of postoperative shivering with dexamethasone: A prospective randomized clinical trial. Iran J Med Sci. 2015;27(1):15–7.
37. Entezariasl M, Isazadehfar K. Dexamethasone for prevention of postoperative shivering: a randomized double-blind comparison with pethidine. Int J Prev Med. 2013 Jul;4(7):818–24. PMID:24049601
38. Murphy GS, Sherwani SS, Szokol JW, Avram MJ, Greenberg SB, Patel KM, et al. Small-dose dexamethasone improves quality of recovery scores after elective cardiac surgery: a randomized, double-blind, placebo-controlled study. J Cardiothorac Vasc Anesth. 2011 Dec;25(6):950–60. https://doi.org/10.1053/j.jvca.2011.03.002 PMID:21565530
39. Hosseinzadeh, H., Eydi, M., Dadashzadeh, S., Kolahdouzan, K. H., & Pesyanian, E. (2005). The effect of preoperative hydrocortisone on the rate and severity of post anesthesia shivering.
40. Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg. 2012 Apr;114(4):813–22. https://doi.org/10.1213/ANE.0b013e318247f628 PMID:22344239

 ORCID
ORCID