Raúl Guillén R. MD.1, Jaime A. Espinosa E. MD.1, Yazmín Guillén D. M.Sc.1,*
Recibido: 17-11-2022
Aceptado: 20-12-2022
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 2 pp. 217-221|https://doi.org/10.25237/revchilanestv5206021237
PDF|ePub|RIS
Abstract
A high radial-femoral gradient has been described in complex cardiac surgery patients, but its association with organ dysfunction has not been studied well. The principal objective of this study was to analyze whether a high gradient is a significant risk factor for organ dysfunction. Observational and prospective study. We measured the radial-femoral gradient at sternal closure. The sample was divided in 2 groups: Significant gradient (SG) and Without gradient (WG). We use non-parametric analysis. We analyzed data corresponding to 15 patients. The incidence of SG was 73% (11 patients). The anesthetic time was 540 min in SG group vs 438 min in WG, p = 0.04. We found a positive correlation with lactate and SG group, r2 0.8, p = 0.001. The radial-femoral gradient had a high incidence in this high risk population undergoing cardiac complex surgery. There was a positive correlation between significant gradient and lactate, which suggest that a high gradient probably is an indicator of hypoperfusion. We suggest monitoring patients with two artery lines in complex cardiac surgery for early detection of hypoperfusion.
Resumen
El gradiente radial-femoral elevado ha sido descrito en pacientes sometidos a cirugía cardíaca compleja, su asociación con disfunción de órganos no ha sido bien estudiada. El objetivo principal de este estudio fue analizar si un gradiente alto es un factor de riesgo significativo de disfunción de órganos. Estudio observacional, prospectivo. Medimos el gradiente radial-femoral al cierre esternal. La muestra se dividió en dos grupos: gradiente significativo (SG) y sin gradiente (WG). Usamos análisis no paramétrico. Se analizaron datos correspondientes a 15 pacientes. La incidencia de SG fue del 73% (11 pacientes). El tiempo anestésico fue de 540 min en el grupo SG vs 438 min en el WG, p = 0,04. Se encontró correlación positiva con el grupo lactato y el SG, r2 0,8, p = 0,001. El gradiente radial- femoral tuvo una alta incidencia en esta población de pacientes bajo cirugía compleja cardíaca. Encontramos una correlación positiva entre el gradiente significativo y el lactato, lo que sugiere que un gradiente alto probablemente es un indicador de hipoperfusión. Sugerimos monitorizar a los pacientes con dos líneas arteriales en cirugía cardíaca compleja para la detección temprana de hipoperfusión.
-
Introduction
The principal objective of hemodynamic monitoring in cardiac surgery is to prevent tissue hypoxemia. Monitoring helps with goal-directed therapy, constant evaluation of imbalance between oxygen supply and demand, and anesthetic decision-making in hemodynamic instability[1].
Blood pressure is considered a basic standard of anesthetic monitoring, and invasive blood pressure monitoring is essential for hemodynamic status evaluation. It allows for the evaluation of cardiocirculatory function with the trace of intra-arterial pressure[2], arterial oxygen measurement, frequent blood sampling, and gas exchange interpretation[3].
The most common site for invasive pressure monitoring is the radial artery because it has a low risk of complications. The most frequent complication is temporary arterial occlusion, which occurs in 1.5%-35% of cases, but without serious consequences in approximately 0.09%[4].
A high radial-femoral gradient has been described in critical cardiac surgery patients. There are independent risk factors associated with its development, such as the Parsonet score (p = 0.002), aortic cross-clamping time (p = 0.005)[5], and patient size (p = 0.029)[5], its incidence increases with high use of vasopressors in critically ill patients[6]. Not detecting it can lead to underestimation of the central pressure and use of the wrong therapeutic strategy.
Studies have not examined the association between a high radial-femoral gradient and postoperative complications. We wanted to know whether a high gradient has an association with organ failure. Thus, the principal objective of this study was to analyze whether a high radial-femoral gradient is a significant risk factor for organ failure in patients undergoing cardiac complex surgery.
-
Methods
We conducted an observational, prospective, and longitudinal study using the STROBE reporting guidelines. This research was done at a third-level hospital that treats patients with cardiovascular pathologies. The surgery area has 5 surgery rooms for program surgery and 1 surgery room for cardiovascular urgencies. We attend to almost 200 to 300 surgeries with cardio- pulmonary bypass (CPB) per year.
We obtained approval for the study from the ethics and research committee in our institution. Informed consent was not required due to the observational nature of the study. We recollected data from March 2019 to February 2020. All patients were undergoing complex cardiac surgery with CPB. We excluded patients with congenital pathology and if some of their invasive artery pressure failed.
The quantitative variables recorded were blood pressure radial and femoral, age, weight, height, body mass index (BMI), body surface area (BSA), CPB time (CPBt), aortic cross-clamp time (ACCt), surgery time (Qxt), anesthetic time (Anest), basal creatinine, 24 h creatinine, basal lactate, and 24 h lactate. The qualitative variables recorded were gender, surgery, amine use, and mortality. The dependent variable was the development of organ failure, which was defined as one of the following: acute kidney injury in the first 24 h (AKI) according to the “Kidney
Disease Improving Global Outcomes” (KDIGO) classification[7]; hyperlactatemia (> 3 mmol/L) in the first 24 h and prolonged amine use in the first 24 h.
We defined significant gradient according to the criteria re- ported by Fuda et. al,[5]: > 25 mmHg in systolic and > 10 mmHg in the medial radial-femoral pressure gradient. The time that we measured the gradient was 10 minutes after sternal closure. Complex cardiac surgery was defined as patients undergoing triple valvular substitution, coronary bypass with > 3 bridges in combination with double or triple valvular substitution, aneurism aortic surgery repair (Bentall or David procedures), valvular substitution in re-operated patients and pulmonary thrombo- endarterectomy.
All data were obtained prospectively. The anesthesiologist in charge placed a catheter (Arrow 20 Ga®) in the radial position before anesthetic induction, and another catheter (Arrow 20 Ga®) was placed in the femoral artery after anesthetic induction on a routine basis. Both pressures were monitored with a trans- ducer (TranStar Medex®). We visualized both pressure curves on a monitor (Carescape B650, Datex-Ohmeda Avance CS2®). The transducers were calibrated to zero before recording data. To reduce the risk of bias, only one investigator recorded the measurements, and data were recorded as the mean of three consecutive measurements.
The sample size was calculated with an exposed probability of a high gradient of 60%, a relative risk (RR) estimation of 3, a confidence level of 95%, a z value of 1.96 and a statistical power of 80%. The sample size calculated was 20 patients.
We included 20 patients but 5 patients were eliminating secondary to dysfunction of one artery line in the principal surgery time then we evaluate values of arterial pressures corresponding to 15 patients. According to the Shapiro-Wilks test, the distribution of data was not Gaussian, and we employed non-parametric tests. The quantitative variables were divided into two groups: significant gradient (SG) and without gradient (WG). The results were expressed using the medians, minimal, maximal, and interquartile ranges. To examine the difference between groups, we used the Mann-Whitney U test. Qualitative variables were expressed with frequencies and percentages, and the differences between them were analyzed with the X2 test. We considerad statistical valúes to be significant at p < 0.05. To evaluate the association of SG with organ failure we used a univariate analysis. We used SPSS version 21 for Windows, and we did not lose data.
-
Results
The SG was observed in 11 patients (73%), there was no significant difference between groups in terms of age, weight, height, BMI, BSA, CPBt, ACCt, Qxt, or gender. The median Anest was 540 minutes (360-800, IR 440) in the SG group and 438 minutes (420-492, IR 72) in the WG group, p = 0.04 statistically significant. The median postoperative lactate was 3.2 mmol/L (1.9-13.2, IR 11.3) in the SG group and 2.5 mmol/L (1.3-3, IR 1.7) in the WG group, p = 0.02 statistically significant (Table 1).
The gradient showed no significance at the beginning of the monitoring but at sternal closure the gradient was significant in systolic and mean pressure (Table 2).
Table 1. Demographic data
| Significant gradient n = 11 | Without gradient n = 4 | P* | |
| Age (years) | 45 (23-69,46) | 56 (38-70,32) | 0.22 |
| Weight (kg) | 74 (49-95,46) | 66 (57-95,38) | 0.85 |
| Height (m) | 1.67 (1.43-1.95,0.52) | 1.62 (1.52-1.79,0.27) | 0.57 |
| BMI (kg/m2) | 25.91 (19.14-30.77,11.63) | 26.39 (22.30-29.64,7.34) | 0.94 |
| BSA (m2) | 1.81 (1.4-2.27,0.87) | 1.70 (1.52-2.13,0.61) | 1 |
| CPBt (min) | 191 (97-374,277) | 157 (125-234,109) | 0.57 |
| ACCt (min) | 149 (68-236,168) | 105 (102-128,26) | 0.45 |
| Anest (min) | 540 (360-800,440) | 438 (420-492,72) | 0.04* |
| ***Creatinine (g/dL) | 1.17 (0.71-1.64,0.92) | 0.95 (0.56-1.22,0.65) | 0.30 |
| ***Lactate (mmol/L) | 3.2 (1.9-13.2, 11.3) | 2.5 (1.3-3, 1.7) | 0.02* |
| Male (%) | 3 (20) | 2 (13.3) | 0.56 |
| Female (%) | 8 (53.3) | 2 (13.3) | |
| Data = Median (minimal-maximum, Intercuartil range); *Mann-Whitney U test, p < 0.05 statistically significant; ***Postoperative. BMI, Body Mass Index; BSA, body surface area; CPBt, cardiopulmonary bypass time; ACCt, aortic cross clamping time; Anest, anesthetic time. | |||
Table 2. Radial-Femoral Gradient in basal and sternal closure time
| Significant gradient n = 11 | Without gradient n = 4 | P | |
| Basal | P | ||
| SRBP (mmHg) | 92 (64-135,71) | 102 (70-157,87) | 0.476 |
| SFBP (mmHg) | 105 (80-130,50) | 109 (84-159,75) | 0.381 |
| Systolic Gradient radial-femoral (mmhg) | 11 (-5-20,25) | 4 (-2-23,25) | 0.019 |
| MRBP (mmHg) | 71 (64-98,34) | 68 (46-104,58) | 0.381 |
| MFBP (mmHg) | 73 (62-98,36) | 70 (47-112,65) | 0.305 |
| Mean Gradient radial-femoral (mmHg) | 3 (-4-5,9) | 1 (-5-8,13) | 0.019 |
| Sternal closure | |||
| SRBP (mmHg) | 62 (61-63,2) | 103 (83-134,51) | 0.004 |
| SFBP (mmHg) | 11 1 (89-1 12,23) | 119 (96-137,41) | 0.295 |
| Systolic Gradient radial-femoral (mmHg) | 48 (28-50,22) | 12.5 (2-20,18) | 0.004 |
| MRBP (mmHg) | 58 (39-86,47) | 71 (59-82,23) | 0.226 |
| MFBP (mmH was not g) | 74 (63-102.39) | 70 (63-86.23) | 0.489 |
| Mean Gradient radial-femoral (mmHg) | 13 (10-29,19) | 2 (-3-5,8) | 0.001 |
| Data = median (minimal – maximal, interquartile range), p < 0.05 statistically significant, U de Mann Whitney Test; SRBP systolic radial blood pressure; SFBP systolic femoral blood pressure; MRBP mean radial blood pressure; MFBP mean femoral blood pressure; P < 0.05 statistically significant; Significant gradient: systolic > 25 mmHg and mean > 10 mmHg. | |||
The R Pearson correlation between SG and lactate was positive, r2 0.8, p = 0.001 statistically significant, if a gradient increase the lactate increase too (Figure 1). We did not find an association with other complication (Table 3).
-
Discussion
We found an association between SG and hyperlactatemia in the first 24 h (r2 0.8, p = 0.001). The incidence of SG was
73%. We did not find an association with SG and AKI, death, or prolonged amine use. Our incidence was consistent with the literature, Fuda et al., reported an incidence of 45%[5], and Manecke et al[8], reported a high incidence of 76% with the same gradient definition.
Some factors associated with SG have been the ACCt (p = 0.005)[5], short height (p = 0.029)[5], age more than 65 years old (p = 0.05)[8] and vasoconstriction[9],[10]. In our study the anesthetic time was high, 540 min (360-800 min, IR 440) in SG group in relation to WG group, p = 0.04.
Table 3. Complications
| Significant gradient n = 11 | Without gradient n = 4 | P | |
| Dead Yes | 2 (13.3%) | 1 (6.7%) | 0.08 |
| No | 1 (6.7%) | 11 (73.3%) | |
| AKI Yes | 1 (6.7%) | 2 (13.3%) | 0.51 |
| No | 2 (13.3%) | 10 (66.7%) | |
| Prolonged amine use Yes | 1 (6.7%) | 1 (6.7%) | 0.37 |
| No | 2 (13.3%) | 11 (73.3%) | |
| Data in Frequencies (percentages). Fisher Exact Test, *p<0.05 statistically significant; AKI Acute Kidney Injury. | |||
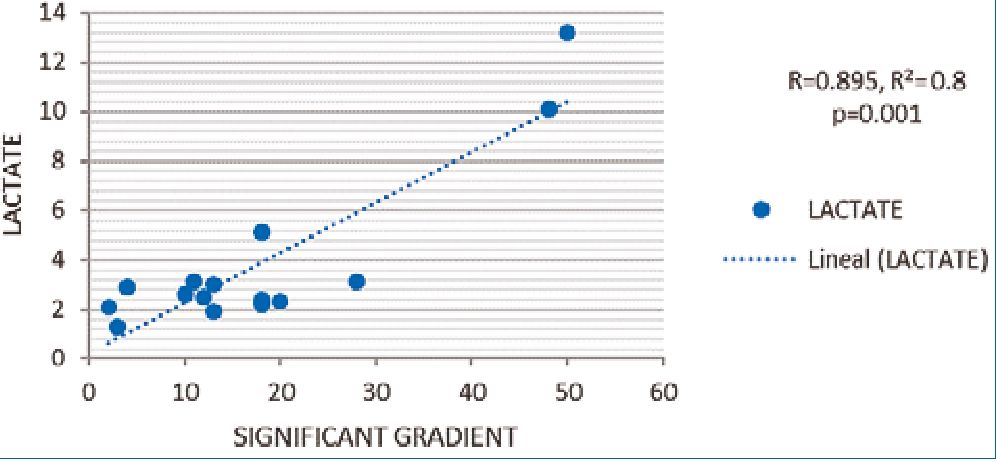
Figure 1. R Pearson Correlation between lactate and significant gradient.
The etiology of vasoconstriction has been controversial. Baba et. al[9], reported a reduction in the diameter of the radial artery after CPB without a significant increase in the artery flow rate, which could explain the high gradient. Kanazawa et. al[12], found that intra-arterial pressure and pulse wave velocity gradually decrease from the aorta to the radial artery, but they were not able to clarify whether there is a component of change in the elasticity of the vessels. Antal et. al[13], did not find an association with vasopressor use. This study did not find significant association with vasopressors use in SG patients.
On the other hand, vasodilation has been associated with the development of the gradient as a pathophysiological mechanism in cardiac surgery after CPB probable to the release of pro-inflammatory cytokines, activation of the endothelium and release of vasoactive substances such as nitric oxide and prostacyclins[14], however, in our study we did not find the use of persistent vasoconstrictor amines although we cannot rule out this phenomenon due to the lack of specific measurements of resistance or arterial size by ultrasound which limits ruling out this phenomenon.
Early hypoperfusion detection is a principal objective in critical care management. Lactate is an early indicator of organ dysfunction; in cardiac surgery the hyperlactatemia can be explained by microcirculatory dysfunction secondary to increased anaerobic metabolism triggered by an increase in circulating inflammatory proteins[11]. In this population we had prolonged anesthetic times in the SG group maybe, it can explain the high lactate levels in SG group in relation to WG group secondary a high risk of tissue hypoperfusion in a group of complex surgery, a high gradient could be a surrogate of hypoperfusion because in our case the correlation was positive indicating that with an increase in the gradient and increase in lactate probably occurred.
More studies are needed to determine the association be- tween hypoperfusion and high pressure gradient however, these results suggest that is important the monitoring of central and peripheral pressures in critically ill patients undergoing complex cardiac surgery for detection of high gradient and its probably association to hypoperfusion.
-
Conclusions
In conclusion, the high radial-femoral gradient had a high incidence in patients undergoing complex cardiac surgery. There was a positive correlation with the SG and hyperlactatemia in the first 24 h. Thus, we suggest that SG increases the risk of hypoperfusion. Early detection of SG could guide maneuvers to improve perfusion. Our study was not able to detect an association with other types of relevant complications.
Funding sources: This research did not receive any specific grant from funding in the public, commercial, or not-for-profit sectors.
Conflict of Interest: non.
-
References
1. Arora D, Mehta Y. Recent trends on hemodynamic monitoring in cardiac surgery. Ann Card Anaesth. 2016;19(4):580–3. https://doi.org/10.4103/0971-9784.191557 PMID:27716684
2. Carl M, Alms A, Braun J, Dongas A, Erb J, Goetz A, et al. S3 guidelines for intensive care in cardiac surgery patients: hemodynamic monitoring and cardiocirculary system. Ger Med Sci. 2010 Jun;8:Doc12. https://doi.org/10.3205/000101 PMID:20577643
3. Tegtmeyer K, Brady G, Lai S, Hodo R, Braner D. Videos in Clinical Medicine. Placement of an arterial line. N Engl J Med. 2006 Apr;354(15):e13. https://doi.org/10.1056/NEJMvcm044149 PMID:16611944
4. Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002 Jun;6(3):199–204. https://doi.org/10.1186/cc1489 PMID:12133178
5. Fuda G, Denault A, Deschamps A, Bouchard D, Fortier A, Lambert J, et al. Risk Factors Involved in Central-to-Radial Arterial Pressure Gradient During Cardiac Surgery. Anesth Analg. 2016 Mar;122(3):624–32. https://doi.org/10.1213/ANE.0000000000001096 PMID:26599795
6. Kim WY, Jun JH, Huh JW, Hong SB, Lim CM, Koh Y. Radial to femoral arterial blood pressure differences in septic shock patients receiving high-dose norepinephrine therapy. Shock. 2013 Dec;40(6):527–31. https://doi.org/10.1097/SHK.0000000000000064 PMID:24089010
7. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. https://doi.org/10.1159/000339789 PMID:22890468
8. Manecke GR Jr, Parimucha M, Stratmann G, Wilson WC, Roth DM, Auger WR, et al. Deep hypothermic circulatory arrest and the femoral-to-radial arterial pressure gradient. J Cardiothorac Vasc Anesth. 2004 Apr;18(2):175–9. https://doi.org/10.1053/j.jvca.2004.01.023 PMID:15073707
9. Baba T GT, Yoshitake A, Shibata Y. Radial artery diameter decreases with increased femoral to radial arterial pressure gradient during cardiopulmonary bypass. Anesth Analg. 1997;85(2):252- https://doi.org/10.1097/00000539-199708000-00003.. PMID: 92490968.
10. Bouchard-Dechêne V, Couture P, Su A, Deschamps A, Lamarche Y, Desjardins G, et al. Risk Factors for Radial-to-Femoral Artery Pressure Gradient in Patients Undergoing Cardiac Surgery With Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth. 2018 Apr;32(2):692–8. https://doi.org/10.1053/j.jvca.2017.09.020 PMID:29217231
11. Minton J, Sidebotham DA. Hyperlactatemia and Cardiac Surgery. J Extra Corpor Technol. 2017 Mar;49(1):7–15. PMID:28298660
12. Kanazawa M, Fukuyama H, Kinefuchi Y, Takiguchi M, Suzuki T. Relationship between aortic-to-radial arterial pressure gradient after cardiopulmonary bypass and changes in arterial elasticity. Anesthesiology. 2003 Jul;99(1):48–53. https://doi.org/10.1097/00000542-200307000-00011 PMID:12826841
13. Antal O, Ştefănescu E, Hagău N. Does norepinephrine infusion dose influence the femoral-to-radial mean arterial blood pressure gradient in patients with sepsis and septic shock? Blood Press Monit. 2019 Apr;24(2):74–7. https://doi.org/10.1097/MBP.0000000000000363 PMID:30681421
14. Jacquet-Lagrèze M, Costescu A, Denault A. Can we trust radial artery pressure monitoring for cardiac surgery? Can J Anaesth. 2022 Nov;69(11):1319-1326. English. https://doi.org/10.1007/s12630-022-02321-1 PMID: 36198991.

 ORCID
ORCID

 Creative Commons Attribution
Creative Commons Attribution