Ismail Mohammed Ibrahim MD. 1,* , Mohamed Mohsen Mohamed Awad Rashed MD. 1 , Tamer Samir Abdelsalam Abdelaziz MD. 1
Recibido: 01-04-2022
Aceptado: 22-10-2022
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 1 pp. 59-67|https://doi.org/10.25237/revchilanestv5207111210
PDF|ePub|RIS
Comparación entre el uso de dexametasona sulfato de magnesio dexmedetomedina con bupivacaina en el bloqueo del canal del abductor bajo ultrasonido para analgesia de repación artoscópica de ligamento cruzado
Abstract
Arthroscopic knee surgery refers to different surgical interventions in the knee joint and many studies investigated postoperative pain control, Adductor canal block (ACB) as a new alternative technique to femoral nerve block (FNB) provides post-operative pain control with a multimodal analgesic regimen after knee surgery with better quadriceps muscle strength, Many adjuvants have been added to local anesthet- ics to prolong the analgesic duration of ACB like dexamethasone and dexmedetomidine. A prospective, randomized, double-blind, parallel-four groups trial. A total of 120 patients scheduled for elective ACL repair; American society of anesthesiologists- physical status I and II patients, aged 18 to 60 years, 155-170 cm height, both sexes. Patients were randomized into four groups dexamethasone group (DX)group in which dexamethasone was added to bupivacaine in ACB, dexmedetomidine (Dm) group in which dexmedetomidine added to bu- pivacaine in ACB, magnesium sulfate (m) group in which magnesium sulfate was added to bupivacaine in ACB and control group with normal saline added to bupivacaine in ACB. The main outcome measure was the duration of postoperative analgesia. The results revealed that the addition of 8 mg dexamethasone or 25 pg dexmedetomidine to 0.5% bupivacaine solution during ACB improved the duration and the quality of postoperative analgesia but the addision of magnesium sulfate had no role. We concluded that the addition of dexamethasone or dexmedetomidine to bupivacaine in ACB after arthroscopic ACL repair under spinal anesthesia provided longer postoperative analgesia and less analgesic consumption.
Resumen
La cirugía artroscópica de rodilla incluye diferentes procedimientos dolorosos sobre la articulación lo que genera diversos estudios. El bloqueo del canal de los abductores es una nueva alternativa al bloqueo femoral que junto al uso de analgesia multimodal logra buen efecto con menos compromiso del cuádriceps. Se han sumado diversos adjuvantes, como dexametasona y dexmedetomidina y así prolongar el efecto analgésico del bloqueo. En forma prospectiva, randomizada, doble ciego y paralela en 4 grupos se comparó un total de 120 pacientes ASA 1 y 2, edad 18 a 65 años,entre 1,55 m a 1,70 m y de ambos géneros sometidos a cirugía artoscopica electiva de reparación del ligamento cruzado. Los pacientes fueron randomizados en 4 grupos: Grupo DX: Dexmedetomidina se agregó a bupivacaina; Grupo Dm: Dexametasona se agregó a bupivacaina; Grupo m: Sulfato de magnesio se agregó a bupivacaina; Gripo control: se agregó solución fisiológica a bipivacaina. El objetivo primario fue evaluar la duración de la analgesia. El uso de 8 mg de dexametasona y el uso de 25 gammas de Dexmedetomidina prolongó la analgesia postoperatoria, no así el sulfato de magnesio. Agregar dexametasona y/o dexmedetomidina a la bupivacaina en un bloqueo del canal de los abductores prolonga su efecto analgésico y disminuye el uso de fármacos de rescate.
-
Introduction
Arthroscopic anterior cruciate ligament (ACL) reconstructions are frequently performed as a day case procedure. However, they are associated with moderate to severe postoperative pain that may prevent same-day discharge. Different analgesic methods have been investigated, including systemic and intra-articular analgesics and neuraxial and peripheral nerve blocks[1].
Early mobilization is essential for reducing post-operative immobility complications and for the best functional outcomes. Postoperative pain control improves rehabilitation, facilitates physiotherapy, increases patient satisfaction, and decreases hospital stay[2].
Numerous pain control modalities have been used but with different undesired effects; like Femoral nerve block (FNB) which showed a better pain relief than narcotic patient-con- trolled analgesia (PCA), but with risk of fall and delayed mobilization[3]. Adductor canal block (ACB) as a new alternative technique to FNB provides post-operative pain control with a multimodal analgesic regimen after knee surgery with better quadriceps muscle strength[4],[5].
Multimodal analgesia includes systemic (opioid and non- opioid) analgesics and regional (neuraxial and peripheral) nerve blocks that are combined to provide pain relief through synergistic actions at different sites and different mechanisms in pain pathways with lower side effects. Postoperative analgesia with motor preservation and early mobilization are considered the main goal following knee arthroscopy for proper physiotherapy and early recovery[6].
Epidural analgesia provides pain relief with motor block and urine retention[7], whereas FNB has a high risk of falls due to decreased quadriceps muscle strength[8].
Vander Wal has described adductor canal block as an approach to block the saphenous nerve with better preservation of quadriceps muscle strength than FNB[9],[10].
Many adjuvants have been added to local anesthetics to prolong the analgesic duration of ACB like dexamethasone, dexmedetomidine[11], and magnesium sulfate[12].
We hypothesize that dexamethasone, dexmedetomidine, or magnesium sulfate will prolong the postoperative analgesic effect of adductor canal block when added to bupivacaine following anterior cruciate ligament repair and will decrease the total requirements of postoperative narcotics.
-
Materials and Methods
This was a prospective randomized parallel-groups, nonfunded, single-center study (Ain Shams University Hospital) conducted after institutional ethics committee approval. All procedures performed in the study involving human participants followed the ethical standards of the institutional re- search committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The work was approved by the Ethics committee of the University hospital (FMASU R 87/ 2021) on 8/4/2021. It also was registered at Clinical Trial Registry ClinicalTrials.gov Identifier: NCT04892420. Following WHO and ICMJE standards. Written informed consent was obtained from all patients. This trial followed the CONSORT statement, 120 American society of anesthesiologists- Physical status (ASA-PS) I and II patients, aged 18 to 60 years, 155-170 cm height, both sexes, undergoing anterior cruciate ligament under spinal anesthesia were included in the study.
The study excluded patients who Declined to give written informed consent, patients with a history of allergy to the medications used in the study, patients with contraindications to regional anesthesia (including coagulopathy and local infection), and patients with a neuropsychiatric disorder, diabetes mellitus, renal and hepatic dysfunction emergency surgery.
Adult patients undergoing anterior cruciate ligament repair under Spinal Anesthesia were randomly assigned into one of the following groups (The four study groups were received the standard treatment in the form of spinal anesthesia and adductor canal block). Group DX (Dexamethasone): The patients received 20ml plain bupivacaine (0.5%) + 8 mg dexamethasone (2ml) in adductor canal block. Group DM (Dexmedetomidine): The patients received 20ml plain bupivacaine (0.5%) + 25 pg dexmedetomidine (diluted in 2 ml normal saline) in adductor canal block. Group M (Magnesium sulphate): The patients received 20 ml plain bupivacaine (0.5%) + 200 mg magnesium sulphate (2 ml of magnesium 10%) in adductor canal block and Group C (Control): The patients received 20ml plain bupivacaine (0.5%) + 2 ml of Normal saline in adductor canal block.
-
In the period between 9th June and 30th October 2021
Randomization was performed using a computer-generated random number table in opaque sealed envelopes with a 1:1 allocation ratio by an anesthesiologist not directly involved in the trial or patient care. The group allocation list was discreetly shared with the anesthesia technician (not involved in the intraoperative management), who prepared the study drug syringes as per the sequence number and assigned patients to the trial groups. Both the study drugs were prepared in an identical syringe as colorless solutions and provided to the operating room anesthesiologist for administration to ensure blinding. Patients were subsequently followed up by a researcher who was unaware of the group allocation. Thus effectively, the patient, anesthesiologist, and the outcome assessor were blinded to the group allocation.
All patients were clinically evaluated, and routine preoperative investigations were done, including CBC, coagulation profile, liver function tests, kidney function tests, fasting blood sugar, and ECG.
In the operative room, ECG, non-invasive blood pressure, and pulse oximetry were connected and baseline parameters such as systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate (HR), and oxygen saturation (SpO 2 ) were recorded.
An intravenous (IV) line was inserted, and lactated Ringer was infused.
All patients were positioned similarly during the procedure and were operated on by the same team.
Spinal anesthesia was performed under complete aseptic conditions using a spinal needle of 25 Gauge, where hyperbaric 0.5% bupivacaine 20 mg and fentanyl 25 pg were injected.
ACB was done in the study at the end of surgery using a 22 Gauge 100 mm length, short-beveled regional block needle, skin antiseptic solution, sterile gloves, and an ultrasound machine.
Group DX: The patients received 20 ml plain bupivacaine (0.5%) + 8 mg dexamethasone (2 ml).
The leg that underwent surgery was externally rotated, the knee slightly flexed, and the thigh prepared with betadine. The medial aspect of the thigh was scanned in a transverse axial plane using a high-frequency linear probe prepared in a sterile fashion. The probe was placed to obtain a short-axis view of the femoral artery at the mid-femoral level. The saphenous nerve (SN) adjacent to the femoral artery was identified. The femoral artery was followed distally to the point at which it deviates posteriorly into the popliteal fossa. At this point, the saphenous nerve was identified as it continued in its original course just underneath the sartorius muscle. At a distance of no more than 7cm proximal to the medial condyle, a short-axis view of the sartorius and vastus medialis muscles was obtained with the saphenous nerve identified between the two muscles.
The operator used an in-plane approach and advanced the needle from lateral to medial into the adductor canal through sartorius or vastus medialis, aspirated and injected a test dose of 1 ml of the local anesthetic solution, observe the spread of the local anesthetic to ensure your needle tip in the adductor canal. Consider the intravascular placement of the needle if no spread of local anesthetic was seen and reposition the needle, aspirate every 5 ml.
GROUP DM: 25 pg dexmedetomidine was Added.
GROUP M: 200 mg magnesium sulfate was added.
GROUP C: Nothing was added to bupivacaine.
-
Postoperative settings
Visual analog scale (VAS) was used to evaluate the postoperative pain; The visual analog scale (VAS) is a validated, subjective measure for acute and chronic pain, where the patient marks on a 10 cm line that represents a continuum between “no pain” on the left end (0 cm) of the scale and the “worst pain” on the right end of the scale (10 cm).
When VAS > 3 postoperatively, intravenous morphine was given 0.1 mg per kg not to be repeated in less than 3 h, the time of the first request for postoperative analgesia and the number of injections were recorded. Any side effects were recorded as hypotension (systolic arterial pressure < 90 mmHg), arrhythmia, bradycardia (HR < 60 beat/min), nausea and vomiting, or any other complications. Atropine 0.5 mg was given in response to bradycardia, and up to 20 ml per kg lactated Ringer was given in response to hypotension.
If local anesthetic toxicity occurred, cardiovascular and respiratory support and 100 ml intralipid 20% bolus over 2-3 min could be given.
HR and MBP were measured upon arrival to the PACU and after 30 min, then every hour if the patient remained in the PACU.
In the surgical ward, vital signs (HR, SBP, MBP, DBP) as well as pain intensity were assessed every 2 h during the first 6 h and then every 6 h thereafter for 24 h postoperatively. All patients received ketorolac 30 mg IM every 8 h.
The Primary outcome: The duration of postoperative analgesia (the time to the first rescue analgesic request) through the assessment of VAS (ranging from 0 to 10, where 0 no pain and 10 maximum pain) every 2 h during the first 6 h and then every 6 h thereafter for 24 h postoperatively.
-
Secondary outcome
- The total dose of morphine was used postoperatively for 24 h.
- The number of patients requested rescue analgesia.
-
Sample size calculation
Using PASS 11 program for sample size calculation, at a setting power of 99%, significance level of 0.05, and by reviewing previous study results (Hamada et al., 2019)[13], showed that the mean and standard deviation of the duration of sensory block in dexmedetomidine plus bupivacaine versus dexamethasone in upper limb surgeries were (19.0 ± 1.8 versus 12.03 ± 1.54 respectively); Based on that, one hundred twenty (120) patients undergoing anterior cruciate ligament repair surgery were needed (30 patients in each group).
All data were analyzed statistically and were included in the SPSS software version 21. The appropriate statistical method was used for analysis. Descriptive statistics such as mean, standard deviation, and percentages were used. Comparison of categorical data was done using Chi-square test and for continuous data; an unpaired “t” test was used.
-
Results
One hundred sixty-eight patients scheduled for elective anterior cruciate ligament repair under spinal anesthesia were screened for eligibility and 120 met eligibility criteria and were randomly allocated to receive either dexmedetomidine versus dexamethasone versus magnesium sulfate added to bupivacaine in ACB or plain bupivacaine in control group (Figura 1).
We found no significant differences between the four groups in terms of age, gender, and height (Table 1). All the surgical procedures were completed without complications.
There were no significant differences between the four groups in VAS 2, 4, 6 h postoperatively and there were highly significant differences between them in VAS 12, 18, 24 h post- operatively (in post hoc analysis there was no significant difference between DX & DM groups in VAS 12, 18, 24, there were highly significant differences when DX group compared to M group or control groups, there were highly significant differences when DM group compared to M or control groups and there were non-significant differences between M & control groups (Table 2).
There were highly significant differences between the four groups in the number of analgesic requests, time of the first analgesia, and total analgesic requirements postoperatively (in posthoc analysis there were non-significant differences between DX & DM groups there was a highly significant difference when DX group compared either to M group or control groups, there were highly significant differences when DM group compared either to M or control groups and there was a non-significant difference between M and control groups) (Table 3 and Figure 2, 3, 4).
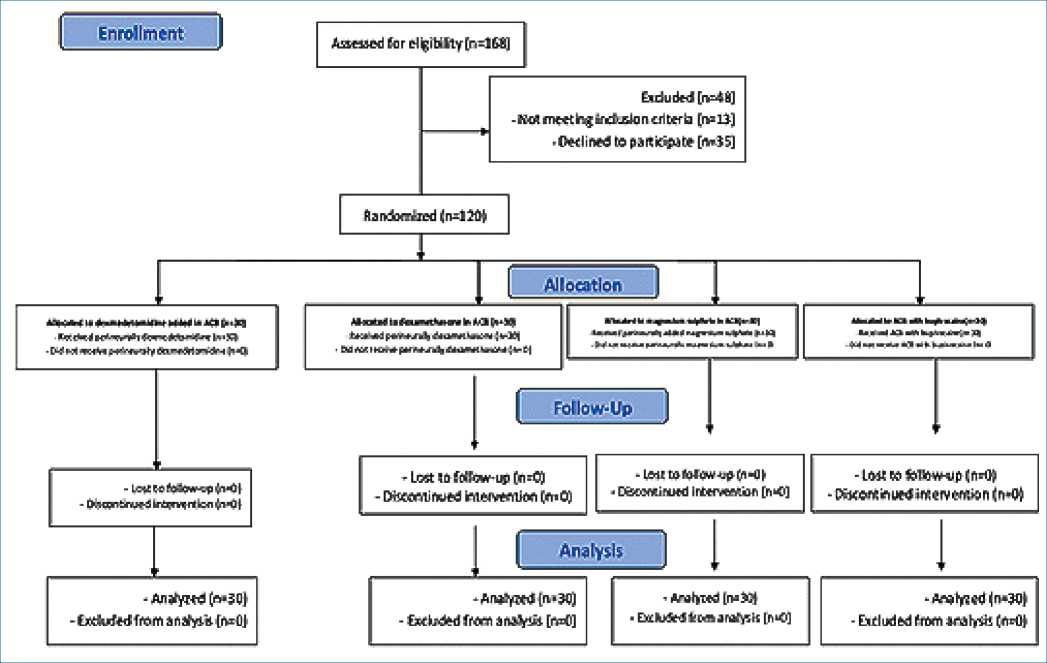
Figure 1. The CONSORT diagram.
Table 1. Difference between 4 groups regarding age, gender, and height
| DX group No. = 30 | DM group No. = 30 | M group No. = 30 | Control group No. = 30 | Test value | P- value | Sig. | |
| Age (years) Mean±SD Range | 37.07 ± 13.21
18 – 64 |
37.10 ± 12.94
18 – 65 |
37.10 ± 12.94
18 – 65 |
37.07 ± 13.21
18 – 64 |
0.000» | 1.000 | NS |
| Sex Female
Male |
15 (50.0%)
15 (50.0%) |
15 (50.0%)
15 (50.0%) |
15 (50.0%)
15 (50.0%) |
15 (50.0%)
15 (50.0%) |
0.000* | 1.000 | NS |
| Height Mean±SD
Range |
164.97 ± 5.76
150 – 170 |
163.30 ± 5.88
155 – 170 |
165.31 ± 5.54
150 – 170 |
163.30 ± 5.88
155 – 170 |
1.021» | 0.386 | NS |
P-value > 0.05: Non significant; P-value < 0.05: Significant; P-value < 0.01: Highly significant.
*: Chi-square test; •: One Way ANOVA test.
-
Discussion
The present study aimed to compare the effect of adding dexamethasone, dexmedetomidine or magnesium sulfate to bupivacaine to improve the efficacy of ACB in patients undergoing anterior cruciate ligament (ACL) repair. The results revealed that the addition of 8 mg dexamethasone or 25 pg dexmedetomidine to 0.5% bupivacaine solution during ACB improved the duration and the quality of postoperative analgesia in patients undergoing ACL repair and reduced the consumption of morphine during the post-operative period, but the Addison of magnesium sulfate had no role on the duration of postoperative analgesia and morphine consumption.
Table 2. Difference between 4 groups in VAS 2, 4, 6, 12, 18 & 24 h postoperatively
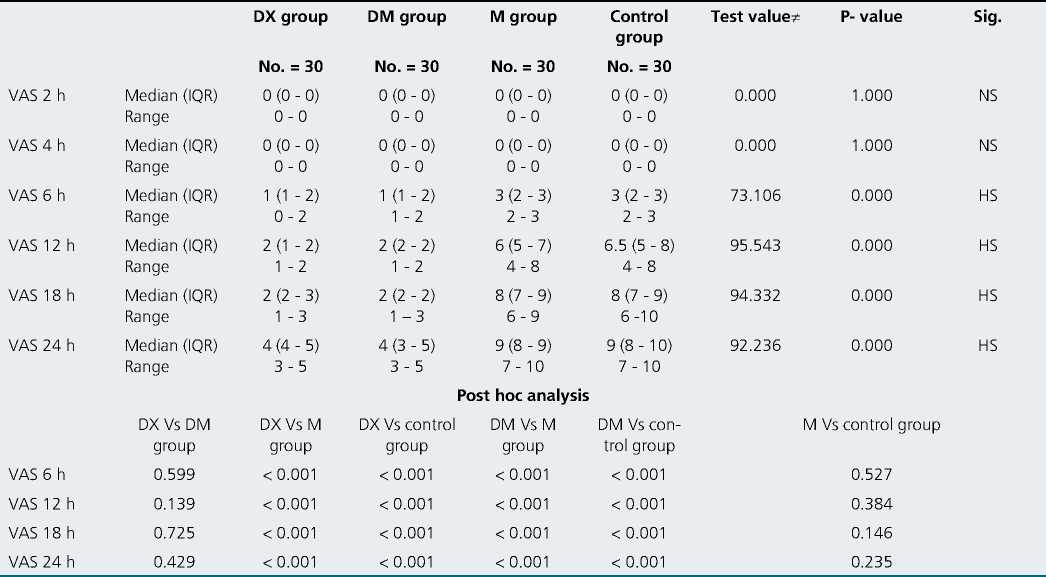
P-value > 0.05: Non significant; P-value < 0.05: Significant; P-value < 0.01: Highly significant.
*: Kruakal-Wallis test.
Table 3. comparison between four groups in analgesia, number of analgesic request, time of first analgesic and total analgesic requirements
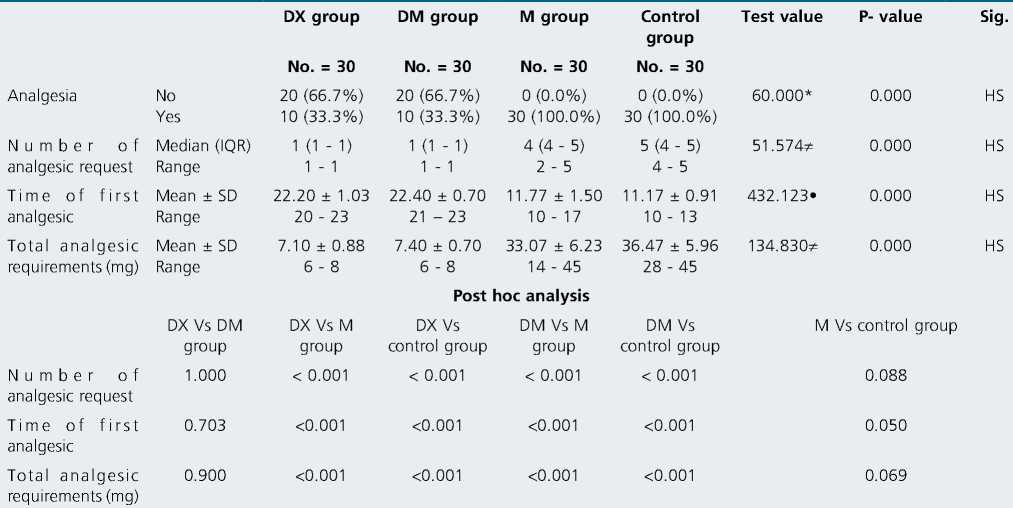
P-value > 0.05: Non significant; P-value < 0.05: Significant; P-value < 0.01: Highly significant.
*: Chi-square test; •: One Way ANOVA test; *: Kruakal-Wallis test.
The addition of 25 pg dexmedetomidine to bupivacaine decreased VAS 12, 18, 24 h postoperatively ((1-2), (1-3) & (3-5) respectively), decreased the percentage of patients’ requests for postoperative analgesia to be 33.3%, decreased the number of analgesic requests (average one time) and prolonged the time of first analgesic request to (22.40 ± 0.7 h) and decreased the total analgesic requirements of morphine to (7.40 ± 0.7 mg).
Bupivacaine 0.5% injection provides approximately 10.8 h of analgesia in ACB (but in our study 11.17 ± 0.91 h), with sparing of quadriceps strength[14]. In an RCT, dexmedetomidine provided analgesia for 18.4hrs when added to 0.75% ropivacaine in an ACB compared to 10.8 h in the control group. Dexmedetomidine as an alpha2-agonist hyperpolarizes pain fibers and inhibits the transmission of nociceptive impulses[15],[16].
Our results are in line with a recent meta-analysis that reported the effectiveness of perineural dexmedetomidine in prolonging the duration of brachial plexus block[16]. Experimental studies on the femoral nerve in dogs[18] and the posterior tibial nerve[19] reported significant prolonged sensory block with the addition of 1.1 pg/ kg dexmedetomidine. A recent study showed a 75% increase in the duration of analgesia with a 100 pg single dose of perineural dexmedetomidine as an adjuvant to femoral and sciatic nerve blocks[20].
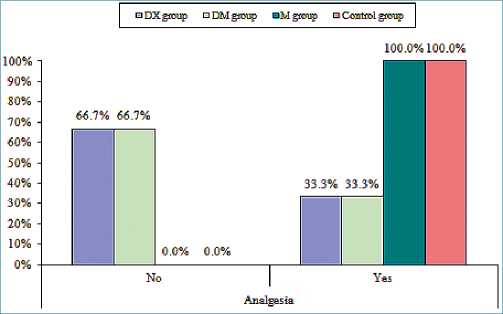
Figure 2. Comparison between four groups in percentage of patients requesting analgesic.
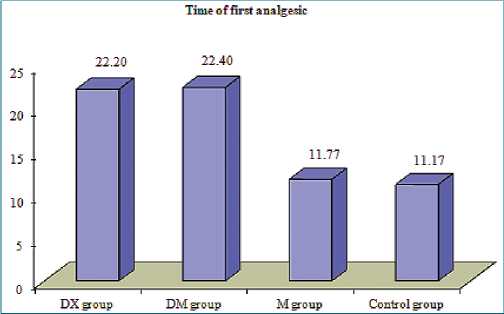
Figure 3. Comparison between four groups in the time of first analgesic.
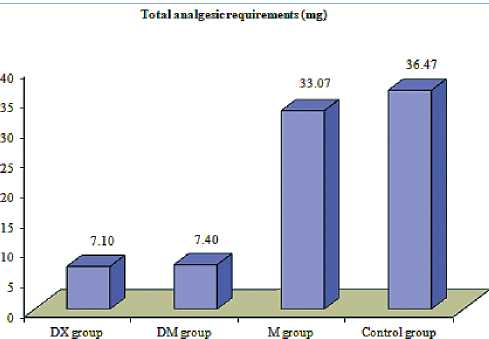
Figure 4. Comparison between four groups in total analgesic requirements.
Most studies used empirical single doses of perineural dexmedetomidine from 20 pg to 150 pg[21],[22]. The only dose-response study for the use of dexmedetomidine was conducted in volunteers for ulnar nerve block as an adjuvant to local anesthetic and concluded that the 100 pg was considered an optimal balance be- tween efficacy and sedation[23].
Dexamethasone has been used to prolong the duration of local anesthetics. In our study dexamethasone decreased VAS 12, 16, 24 h postoperatively ((1-2), (1-3) & (3-5) respectively, decreased the need for postoperative analgesia (only in 33.3% of patients), de- creased the number of analgesic request (average only once per 24 h postoperatively), prolonged the time of first analgesic request to be 22.20 ± 1.03 h and decreased the total analgesic requirements of morphine to be 7.10 ± 0.88 mg.
Dexamethasone reduces the response of small, unmyelinated, and slow conducting C fibers in a dose-dependent manner leading to increased duration of the block[24]. Along with local effects, the systemic effects of dexamethasone may increase the duration of analgesia[25].
Our study agrees with Chisholm et al., the only study to our knowledge that discussed the role of dexamethasone as a peri- neural adjuvant for saphenous nerve blocks in ACL reconstruction where the addition of dexamethasone 1 mg and 4 mg to local anesthesia significantly increased the duration of a saphenous nerve block (8-13 h)[26].
Bj0rn et al., concluded that injection of 10 mL bupivacaine 0.5% with 1: 200,000 epinephrine and 4 mg dexamethasone in saphenous nerve blocks for postoperative pain control after major ankle surgery increased the duration of sensory block and reduced the total opioid consumption[27].
In agreement with our study, Sherif et al., who studied the effect of dexamethasone as an adjuvant for FNB after knee arthroplasty found that injection of 0.5% bupivacaine with 8mg of dexamethasone followed by a continuous infusion, increased the duration of analgesia about 7 h (25.7 ± 3 h vs 18.8 ± 4 h) with less opioid requirements[28].
In a systematic review of 14 studies involving 1,022 patients, Knezevic et al., concluded that dexamethasone in a brachial plexus block significantly decreased opioid consumption at 24 h and significantly improved postoperative pain scores at 48 h. However, perineural dexamethasone delayed the onset of sensory and motor block and prolonged the duration of motor block[29].
In comparison to our results, Cummings et al., studied the effect of dexamethasone on the duration of interscalene block with ropivacaine or bupivacaine and found that perineural dexamethasone prolonged analgesia duration; however, it did not reduce postoperative opioid consumption over the first 72 h[30]. The likely reasons are that a 72 h consumption is a too long time to expect differences in opioid consumption, particularly when the duration of analgesia does not last all that time; in addition, different analgesic protocols likely affect opioid consumption. Also, in contrast to our results, Fredrickson Fanzca et al., showed that perineural dexamethasone had a minor effect on the quality and duration of bupivacaine sciatic and ankle blocks compared with systemic ad- ministration[31].
The addition of 200 mg magnesium sulfate to bupivacaine in ACB didn’t show any favorable role in VAS scores in the first 24 h, postoperative analgesia (in all patients), several analgesic requests (4-5 times), first analgesic request (11.77 ± 1.5 h) and total morphine consumption (33.07 ± 6.23 mg).
Magnesium (Mg) has antinociceptive effects through NMDA receptors blockade[32]. N-methyl-D-aspartate (NMDA) receptors have a significant role in central nociceptive transmission, modulation, and sensitization of acute pain[33]. NMDA receptors had been identified peripherally in the skin[34], muscles[35], and knee joints, and affect the sensory transmission of noxious signals[36]. Mag- nesium ion blocks the NMDA receptor in its inactive state where the depolarizing activity in nociceptor fibers dislodges it and allows calcium influx into the cells[37].
In contrast to our results, Gunduz et al., reported that the addition of 150 mg magnesium sulfate to prilocaine 5 mg/kg provided a mean sensory blockade of 304 min compared to 196 min with prilocaine alone in axillary block[38]. Lee et al., showed that a mixture of magnesium sulfate and bupivacaine 0.5% increased the duration of analgesia and reduced pain but did not affect post- operative opioid consumption in patients undergoing rotator cuff surgery with an interscalene block[39].
In another study, the addition of 150 mg magnesium sulfate to ropivacaine increased the duration of the sensory block from (290 ± 63 to 456 ± 98min) in supraclavicular block[40].
In line with our results, Dana et al., didn’t find any analgesic benefit of adding magnesium sulfate to ACB in total knee arthroplasty patients under spinal anesthesia[12].
-
Conclusion
We concluded that the addition of dexamethasone or dexmedetomidine to bupivacaine in ACB after arthroscopic ACL repair under spinal anesthesia provided longer postoperative analgesia and less analgesic consumption than bupivacaine alone, with no role for magnesium sulfate.
We recommend further studies to determine the optimal dose of dexamethasone or dexmedetomidine which can be added to bupivacaine in ACB and would be associated with the best analgesic effect and to prolong the postoperative period observation, also we need to compare the effect of perineurally versus intravenously added drugs.
Consent for publication
Written informed consent was obtained from all subjects.
Availability of data and material The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Trial Registration: NCT04892420.
Conflicts of Interest: We declare that there were no conflicts of interest.
References
1. Senthilkumaran S, Tate R, Read JR, Sutherland AG. Intra-articular morphine and bupivicaine for post-operative analgesia in anterior cruciate ligament reconstruction: a prospective randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2010 Jun;18(6):731–5. https://doi.org/10.1007/s00167-009-0912-z PMID:19768454
2. Kampitak W. MD, Tanavalee A*, MD, Ngarmukos S*, MD: etal: Malaysian Orthopaedic Journal, comparison of adductor canal block versus local infiltrations analgesia on postopertative pain and functional outcome after total knee arthroplasty,2018.
3. Jaeger P, Grevstad U, Henningsen MH, Gottschau B, Mathiesen O, Dahl JB. Effect of adductor-canal-blockade on established, severe post-operative pain after total knee arthroplasty: a randomised study. Acta Anaesthesiol Scand. 2012 Sep;56(8):1013–9. https://doi.org/10.1111/j.1399-6576.2012.02737.x PMID:22834681
4. Andersen HL, Gyrn J, Møller L, Christensen B, Zaric D. Continuous saphenous nerve block as supplement to single-dose local infiltration analgesia for postoperative pain management after total knee arthroplasty. Reg Anesth Pain Med. 2013;38(2):106–11. https://doi.org/10.1097/AAP.0b013e31827900a9 PMID:23222363
5. Nader A, Kendall MC, Manning DW, Beal M, Rahangdale R, Dekker R, et al. Single-dose adductor canal block with local infiltrative analgesia compared with local infiltrate analgesia after total knee arthroplasty: a randomized, double-blind, placebocontrolled trial. Reg Anesth Pain Med. 2016;41(6):678–84. https://doi.org/10.1097/AAP.0000000000000494 PMID:27776098
6. Slover J, Riesgo A, Payne A, Umeh U. Modern Anesthesia for Total Joint Arthroplasty. Ann. Orthop. Rheumatol. 2014;2:1026.
7. Fowler SJ, Symons J, Sabato S, Myles PS. Epidural analgesia compared with peripheral nerve blockade after major knee surgery: a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2008 Feb;100(2):154–64. https://doi.org/10.1093/bja/aem373 PMID:18211990
8. Ilfeld BM, Moeller LK, Mariano ER, Loland VJ, Stevens-Lapsley JE, Fleisher AS, et al. Continuous peripheral nerve blocks: is local anesthetic dose the only factor, or do concentration and volume influence infusion effects as well? Anesthesiology. 2010 Feb;112(2):347–54. https://doi.org/10.1097/ALN.0b013e3181ca4e5d PMID:20098137
9. Van der wal M., Lang S.A. and Yip R.W.: Transsartorial approach for saphenous nerve block. Can. J. Anaesth., 40: 542-6. Doi: https://doi.org/10.1007/BF03009739,1993..
10. Grevstad U, Mathiesen O, Lind T, Dahl JB. Effect of adductor canal block on pain in patients with severe pain after total knee arthroplasty: a randomized study with individual patient analysis. Br J Anaesth. 2014 May;112(5):912–9. https://doi.org/10.1093/bja/aet441 PMID:24401802
11. Herman J, Urits I, Eskander J, et al:Adductor Canal Block Duration of Analgesia Successfully Prolonged With Perineural Dexmedetomidine and Dexamethasone in Addition to IPACK Block for Total Knee Arthroplasty. Cureus 12(9): e10566. DOI 10.7759/cureus.10566.2020.
12. Zoratto D, Phelan R, Hopman WM, Wood GC, Shyam V, DuMerton D, et al. Adductor canal block with or without added magnesium sulfate following total knee arthroplasty: a multi-arm randomized controlled trial. Can J Anaesth. 2021 Jul;68(7):1028–37. https://doi.org/10.1007/s12630-021-01985-5 PMID:34041719
13. Hamada Hussein Hamada, Wael Mohamed Elmahdy, Mohamed Adel Ashiry, comparative study between dexmedetomidine and dexamethasone as an adjuvant to bupivacaine in ultrasound guided supraclavicular block in upper limb surgeries, the Egyptian Journal of Hospital Medicine(April 2019) Vol 75 (6), page 3060-3069.
14. Goyal R, Mittal G, Yadav AK, Sethi R, Chattopadhyay A. Adductor canal block for post-operative analgesia after simultaneous bilateral total knee replacement: A randomised controlled trial to study the effect of addition of dexmedetomidine to ropivacaine. Indian J Anaesth. 2017 Nov;61(11):903–9. https://doi.org/10.4103/ija.IJA_277_17 PMID:29217856
15. Memiş D, Turan A, Karamanlioğlu B, Pamukçu Z, Kurt I. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg. 2004 Mar;98(3):835–40. https://doi.org/10.1213/01.ANE.0000100680.77978.66 PMID:14980948
16. Kroin JS, Buvanendran A, Beck DR, Topic JE, Watts DE, Tuman KJ. Clonidine prolongation of lidocaine analgesia after sciatic nerve block in rats Is mediated via the hyperpolarization-activated cation current, not by alpha-adrenoreceptors. Anesthesiology. 2004 Aug;101(2):488–94. https://doi.org/10.1097/00000542-200408000-00031 PMID:15277933
17. Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013 Jun;110(6):915–25. https://doi.org/10.1093/bja/aet066 PMID:23587874
18. Bartel AK, Campoy L, Martin-Flores M, Gleed RD, Walker KJ, Scanapico CE, et al. Comparison of bupivacaine and dexmedetomidine femoral and sciatic nerve blocks with bupivacaine and buprenorphine epidural injection for stifle arthroplasty in dogs. Vet Anaesth Analg. 2016 Jul;43(4):435–43. https://doi.org/10.1111/vaa.12318 PMID:26529670
19. Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012 Oct;115(4):958–62. https://doi.org/10.1213/ANE.0b013e318265bab7 PMID:22826530
29. Helal SM, Eskandr AM, Gaballah KM, Gaarour IS. Effects of perineural administration of dexmedetomidine in combination with bupivacaine in a femoral-sciatic nerve block. Saudi J Anaesth. 2016;10(1):18–24. https://doi.org/10.4103/1658-354X.169469 PMID:26955305
21. Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth. 2013 Mar;110(3):438–42. https://doi.org/10.1093/bja/aes400 PMID:23161360
22. Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39(1):37–47. https://doi.org/10.1097/AAP.0000000000000033 PMID:24317234
23. Keplinger M, Marhofer P, Kettner SC, Marhofer D, Kimberger O, Zeitlinger M. A pharmacodynamic evaluation of dexmedetomidine as an additive drug to ropivacaine for peripheral nerve blockade: A randomised, triple-blind, controlled study in volunteers. Eur J Anaesthesiol. 2015 Nov;32(11):790–6. https://doi.org/10.1097/EJA.0000000000000246 PMID:25695189
24. Koyyalamudi V, Sen S, Patil S, Creel JB, Cornett EM, Fox CJ, et al. Adjuvant Agents in Regional Anesthesia in the Ambulatory Setting. Curr Pain Headache Rep. 2017 Jan;21(1):6. https://doi.org/10.1007/s11916-017-0604-1 PMID:28210917
25. Abdallah FW, Johnson J, Chan V, Murgatroyd H, Ghafari M, Ami N, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomized, triple-arm, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 2015;40(2):125–32. https://doi.org/10.1097/AAP.0000000000000210 PMID:25629321
26. Chisholm MF, Cheng J, Fields KG, et al. Perineural dexamethasone with subsartorial saphenous nerve blocks in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016;•••:1–9. PMID:27075893
27. Bjorn S., Linde F., Nielsen K.K., et al: Effect of Perineural Dexamethasone on the Duration of Single Injection Saphenous Nerve Block for Analgesia After Major Ankle Surgery: A Randomized, Controlled Study. Reg. Anesth. Pain Med., 2017.
28. Sherif AA, Elsersy HE. Dexamethasone as adjuvant for femoral nerve block following knee arthroplasty: a randomized, controlled study. Acta Anaesthesiol Scand. 2016 Aug;60(7):977–87. https://doi.org/10.1111/aas.12750 PMID:27255560
29. Knezevic NN, Anantamongkol U, Candido KD. Perineural dexamethasone added to local anesthesia for brachial plexus block improves pain but delays block onset and motor blockade recovery. Pain Physician. 2015;18(1):1–14. PMID:25675053
30. Cummings KC 3rd, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011 Sep;107(3):446–53. https://doi.org/10.1093/bja/aer159 PMID:21676892
31. Fredrickson Fanzca MJ, Danesh-Clough TK, White R. Adjuvant dexamethasone for bupivacaine sciatic and ankle blocks: results from 2 randomized placebo-controlled trials. Reg Anesth Pain Med. 2013;38(4):300–7. https://doi.org/10.1097/AAP.0b013e318292c121 PMID:23698496
32. Hwang JY, Na HS, Jeon YT, Ro YJ, Kim CS, Do SH. I.V. infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. Br J Anaesth. 2010 Jan;104(1):89–93. https://doi.org/10.1093/bja/aep334 PMID:19933175
33. Woolf CJ. Somatic pain—pathogenesis and prevention. Br J Anaesth. 1995 Aug;75(2):169–76. https://doi.org/10.1093/bja/75.2.169 PMID:7577250
34. Iwatsu O, Ushida T, Tany T, et al. Peripheral administration of magnesium sulphate and ketamine hydrochloride produces hypothesia to mechanical stimuli in humans. J Health Sci. 2002;48(1):69–72. https://doi.org/10.1248/jhs.48.69.
35. Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, et al. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003 Oct;90(4):2098–105. https://doi.org/10.1152/jn.00353.2003 PMID:12815021
36. Lawand NB, Willis WD, Westlund KN. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur J Pharmacol. 1997 Apr;324(2-3):169–77. https://doi.org/10.1016/S0014-2999(97)00072-1 PMID:9145768
37. Dickenson AH. NMDA receptor antagonists as analgesics. In: Fields HL, Liebeskind JC, editors. Progress in pain research and management. Volume I. Seattle: IASP Press; 1994. pp. 173–87.
38. Gunduz A, Bilir A, Gulec S. Magnesium added to prilocaine prolongs the duration of axillary plexus block. Reg Anesth Pain Med. 2006;31(3):233–6. https://doi.org/10.1097/00115550-200605000-00010 PMID:16701189
39. Lee AR, Yi HW, Chung IS, Ko JS, Ahn HJ, Gwak MS, et al. Magnesium added to bupivacaine prolongs the duration of analgesia after interscalene nerve block. Can J Anaesth. 2012 Jan;59(1):21–7. https://doi.org/10.1007/s12630-011-9604-5 PMID:22012543
40. Mukherjee K, Das A, Basunia SR, Dutta S, Mandal P, Mukherjee A. Evaluation of Magnesium as an adjuvant in Ropivacaine-induced supraclavicular brachial plexus block: A prospective, double-blinded randomized controlled study. J Res Pharm Pract. 2014 Oct;3(4):123–9. https://doi.org/10.4103/2279-042X.145387 PMID:25535620

 ORCID
ORCID

