Catalina Cárdenas1, Loreto Rojas1,2, Julio Morales Valenzuela3, Yazmín Ivana Pinos García4, María Teresa Silva Elgueta1, María Kappes5*
Recibido: 04-04-2023
Aceptado: 16-05-2023
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 5 pp. 519-526|https://doi.org/10.25237/revchilanestv52n5-11
PDF|ePub|RIS
Esquemas de vacunación y CoronaVac: ¿Existen diferencias entre adultos y adultos mayores hospitalizados por COVID-19?
Abstract
Background: Insufficient evidence exists for comparing different COVID-19 vaccination schemes with CoronaVac in the adult and older adult populations. Methods: A prospective multicenter study was carried out with patients admitted for hospitalization due to COVID-19. Results: A study of 159 COVID-19 patients found that unvaccinated younger patients had a higher risk of oxygen requirement (RR = 1.3, p = 0.041) and dyspnea(p = 0.002). Critical care hospitalizations and the requirement of Invasive Mechanical Ventilation were less frequent across CoronaVac vaccines than in unvaccinated individuals. CanSino and BNT162b2-vaccines patients presented lower levels of D dimer than those vaccinated with CoronaVac. However, vaccinated individuals younger than 60 years old did not have a different risk of death than unvaccinated when presenting severe COVID-19, indicating insufficient vaccine-induced immunity. Conclusions: CoronaVac has a better response in older adults than in young people, and vaccination status did not significantly differ in young individuals with severe illness. Future studies should examine the effects of different vaccines on different age groups and their long-term follow-up, which can help countries make informed decisions for older adults’ vaccination against COVID-19.
Resumen
Antecedentes: Existe evidencia insuficiente para comparar diferentes esquemas de vacunación COVID-19 con CoronaVac en las poblaciones de adultos y adultos mayores. Métodos: Se realizó un estudio multicéntrico prospectivo con pacientes ingresados para hospitalización por COVID-19. Resultados: Un estudio de 159 pacientes con COVID-19 encontró que los pacientes más jóvenes no vacunados tenían un mayor riesgo de requerimiento de oxígeno (RR = 1,3, p = 0,041) y disnea (p = 0,002). Las hospitalizaciones en cuidados intensivos y el requisito de ventilación mecánica invasiva fueron menos frecuentes en las vacunas CoronaVac que en las personas no vacunadas. Los pacientes vacunados con CanSino y BNT162b2 presentaron niveles más bajos de dímero D que los vacunados con CoronaVac. Sin embargo, los individuos vacunados menores de 60 años no tuvieron un riesgo de muerte diferente al de los no vacunados al presentar COVID-19 grave, lo que indica una inmunidad inducida por la vacuna insuficiente. Conclusiones: CoronaVac tiene una mejor respuesta en adultos mayores que en jóvenes, y el estado de vacunación no difirió, significativamente, en individuos jóvenes con enfermedad grave. Los estudios futuros deberían examinar los efectos de diferentes vacunas en diferentes grupos de edad y su seguimiento a largo plazo, lo que puede ayudar a los países a tomar decisiones informadas para la vacunación de adultos mayores contra COVID-19.
-
Introduction
Up to March 1st 2023, more than 758 million SARS- CoV-2 infections have occurred, while more than 6,8 million people have died because of it. At this time, a total of 13.224.955.795 vaccine doses have been administered[1]. During this crisis, poor and developing countries have suffered most of human and economic loss. For instance, from a worldwide perspective, cumulative cases in the Americas have reached more than 29 percent of reported infections, although their entire population represents less than 15 from total[2]. A clear example of the differences between responses against COVID-19 of industrialized countries and the rest of the world is the investment by the first on experimental vaccine platforms, which were quickly developed and administered within their borders. This, while developing countries had limited access to those first batches of anti-SARS-CoV-2 vaccine platforms, like first-time applied and highly effective, mRNA-based formulations[3]. Notably, conventional vaccines like CoronaVac inactivated SARS-CoV-2 formulation, although conferring lower induction of neutralizing titers than these latter[4], have reported a degree of protection against COVID-19 related deaths[5]. In this regard, Chile, began massive vaccination on February 3, 2021, CoronaVac was the first administrated vaccine, later, BNT162b2, AstraZeneca, and CanSino vaccine platforms were available[6]. Nowadays, Chile has more than 93% of its objective population with a complete vaccine schedule received, while more than 58% have received two additional boost vaccination shots[7]. Notwithstanding that effective herd immunity has been aimed to be achieved through vaccination efforts like this or the natural immunity mounted by convalescents[8], the circulation of SARS-CoV-2 has not stopped[9].
Closely related to this continuous circulation is the occurrence of cumulative mutations in SARS-CoV-2, which have been reported since the still-debated[10],[11] precise beginning of COVID-19 pandemic in Wuhan, China. Mutations in SARS- CoV-2 genomes which can increase their transmissibility, and their associated symptoms or even improve the virus ability to evade vaccine-induced immunity, has challenged our strategies to stop COVID-19 pandemic[12]. Some virus variants have been considered as Variants of Interest (VOI), and Variants of Concern (VOC)[13].
Several SARS-CoV-2 variants have been identified since then [14], like Alpha[15], Beta[16], Gamma[17], and Delta[18] VOCs, Epsilon[19] and Mu[20] VOIs, and more recent Omicron[1] VOC-related viral genomes. These have circulated and replaced ancestral sequences whilst the viral spread continues [9]. Particularly, inherent mutations across VOCs, particularly at spike protein, the immunodominant SARS-CoV-2 glycoprotein and main target of vaccine platforms, have challenged naturally and artificially acquired protection against infection[21],[22],[23].
In Chile, a cohort study was carried out with 10.2 million vaccinated people to evaluate the effectiveness of the CoronaVac vaccine. This study found that the adjusted effectiveness of the vaccine was 65.9% (95% confidence interval [CI], 65.2 to 66.6) for the prevention of COVID-19 and 87.5% (95% CI, 86.7 to 88.2) for prevention of hospitalization[24]. A recent systematic review with meta-analysis that included 51 vaccine effectiveness studies determined that the effectiveness of CoronaVac was 65.7% compared to a BNT162b2 of 91.2% and Moderna of 98.1%[25].
In this study, with the aim of describing comorbidities associated to severe COVID-19 pathology in first vaccinees at southern Chile, we studied, making differences by age, their clinical parameters after being diagnosed and hospitalized due COVID-19 between June and July 2021, while at least Gamma, Delta, Lambda, and Mu SARS-CoV-2 variants were circulating in Chile[7].
-
Methods
-
Study design
This multi-center prospective study analyzed 159 hospitalized COVID-19 patients, recruited between June 24th and July 10th, 2021, in southern Chile. A non-probabilistic sampling was carried out by quotes, including 65 patients from Hospital de Osorno (40.9%), 75 patients from Hospital de Puerto Montt (47.2%), and 19 patients from Hospital de Castro, in Los Lagos Region. Their clinical records were analyzed after each informed consent was signed, identifying each vaccination status and related information. Inclusion criteria considered older than 18 years-old patients who received or not any vaccine against SARS-CoV-2 vaccine. To classify patients as vaccinated, they should have received a last shot from their complete vaccination scheme 15 days before recruitment. After considering the situation of Chile during the recruitment period, namely massive vaccination with CoronaVac inactivated SARS- CoV-2 platform was already authorized, considering CanSino clinical assay has had also approved, and that first batches of BNT162b2 mRNA-based platform were already being administered, two comparative groups were generated. Group 1 was created from individuals with complete vaccination schemes of BNT162b2, CanSino, and CoronaVac platforms and compared to unvaccinated individuals, while group 2 was created from CoronaVac vaccinees and compared to unvaccinated individuals.
When available, laboratory tests (C-reactive protein test, D-dimer test, and lymphocyte count) were performed by each recruitment Hospital.
-
Ethical aspects
This study was approved by the scientific ethics committee on July 7th, 2021, following Beauchamp and Childress, beneficence, no-maleficence, justice, and autonomy principles. Confidentiality and anonymity of patients were ensured. Informed consent was signed by enrolled patients and their legal representatives.
-
SARS-CoV-2 variants determination
The diagnosis of SARS-CoV-2 was made from nasopharyngeal swab samples. For viral RNA extraction, the QIAamp Viral RNA Mini Kit (Qiagen®) was used. For the detection of SARS- CoV-2, the SARS-CoV-2 Allplex Assay Kit (Seegene®) was used.
The detection of mutations Alpha (B.1.1.7 lineages), Beta (B.1.35 lineages), Gamma (P.1 lineages) Delta (B.1.617.2 and AY lineages) Epsilon (B.1.43 and B.1.43) Eta (B.1.52) Iota
(B.1.53) Kappa (B.1.617.1) 1.617.3 Mu (B.1.621, B.1.621.1) Zeta (P.2) was made using Allplex™ kits SARS-CoV-2 Variants I (Seegene®), Assay, Allplex™ SARS-CoV-2 Variants II Assay (Seegene®), Allplex™ SARS-CoV-2 Variants IV Assay (Seegene®) and Allplex™ SARS-CoV- 2 Variants VII Assay (Seegene®). Data were tabulated, analyzed, and correlated with clinical information.
-
Statistical analysis
Information analysis was carried out through descriptive measures and the construction of frequency tables. Comparations between groups were done with Z test, for proportions differences, respecting cases number by categories (np>5), and the association and relative risk calculation throughout Pearson’s Chi-square or Fisher’s exact test. Hypotheses were unilaterally evaluated with <0.05 significance. IBM® SPSS Statistic 20,0 SPSS statistical software was used.
-
Results
159 severe COVID-19 patients were recruited, which were hospitalized across southern Chile.
Of them, 89 were men (56%) and 70 were women (44%). Overall average age was 57.7 ± (17.0) years old, which was similar for men (59.0 ± 16.0 years old) and women (56.0 ± 18.0), p=0.359. The most representative age group was elderly, with a 21.4% of total ranging between 60 and 70 years old, while a 27.7% had more than 70 years old at the time of recruitment. This, and other sociodemographic characteristics, symptoms, co-morbidities, and clinical complications of patients were registered and displayed in Table 1. We found that 39.6% of patients received their complete vaccination scheme of CoronaVac shots, while 3.8% of patients already had a complete vaccination scheme of CanSino or BNT162b2 formulations. On the other hand, 47.2% of patients have not received any vaccine shot against SARS-CoV-2 infection, and 5.7% have received incomplete vaccination schedules.
Regarding symptoms, coughing and dyspnea were the most frequent (100 and 111 individuals, representing 62.9 and 69.8%, respectively). Notably, patients who reported dyspnea were less frequent across CoronaVac vaccines than in unvaccinated individuals (p = 0.002). In respect to co-morbidities, while hypertension, Mellitus diabetes, and obesity were the most frequent, only the first two were more prevalent across CoronaVac vaccines than in the group of unvaccinated individuals, p = 0.002 and p = 0.009, respectively.
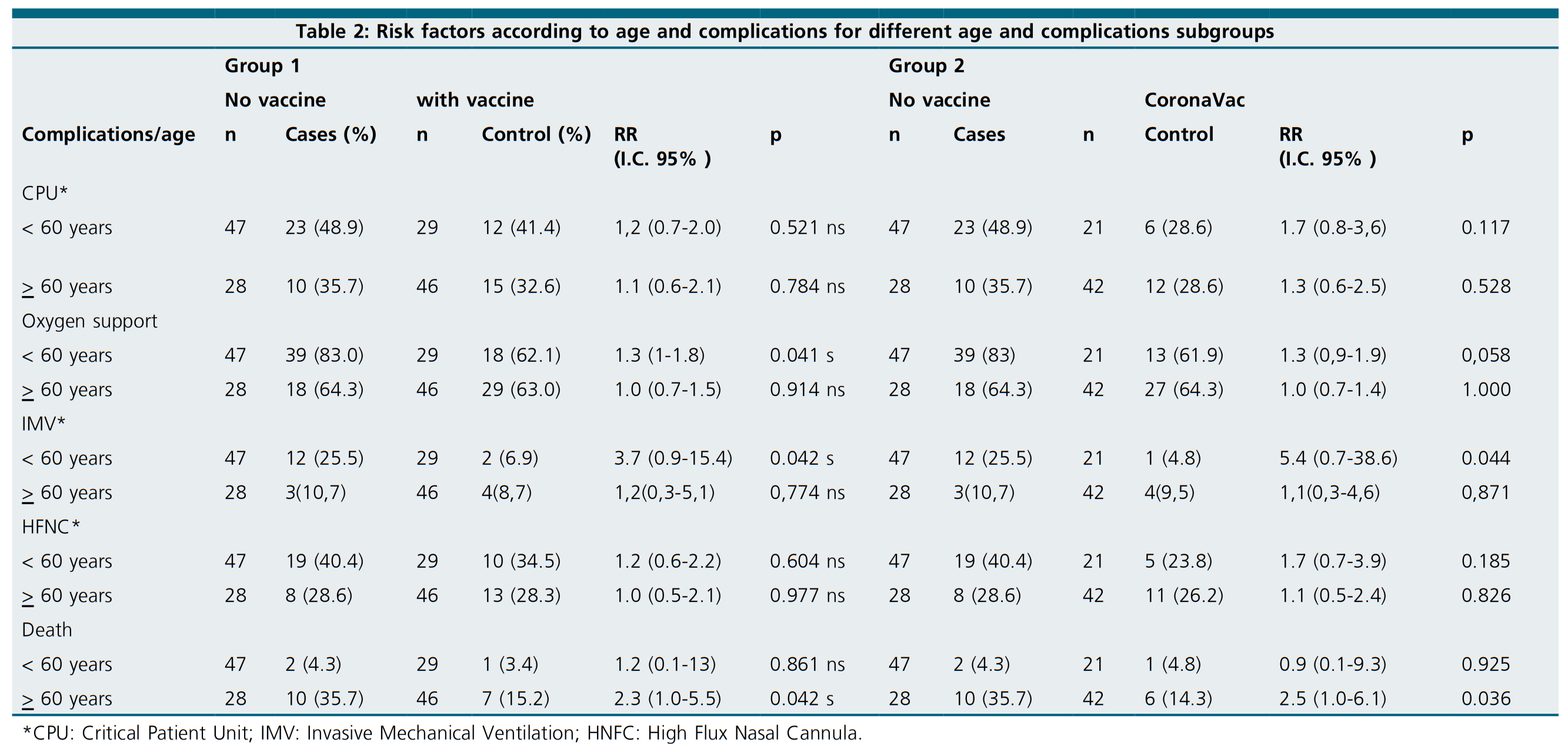
Clinical complications at admission were also recorded. While most patients required oxygen support (67.3%), 39.0% were immediately admitted to the Critical Patients Unit (CPU), and 32.7% were supported with a High Flux Nasal Cannula (HNFC). CPU hospitalizations and the requirement of Invasive Mechanical Ventilation (IMV) were less frequent across CoronaVac vaccines than in unvaccinated individuals (p = 0.028 and p = 0.018, respectively) (Table 2).
To evaluate the effect of vaccination, whichever complete formulation scheme was received, age was used to segregate patients. After grouping patients by being older, our younger than 60 years old, unvaccinated individuals of the younger group had a higher risk for oxygen requirement than fully vaccinated individuals (RR=1.3, p=0.041). Among the same age group, IMV requirement risk was almost 4 times higher across unvaccinated individuals than vaccinated (RR=3.7, p=0.042). When the group of individuals older than 60 years old was analyzed, unvaccinated sub-group had a higher risk of death than vaccinated (RR = 2.3; p = 0.042) (Table 2).
To evaluate the effect of a particular vaccination scheme, CoronaVac, the most representative fully administered formulation, was compared to unvaccinated individuals after agesegregation. Notably, IMV risk was almost 6 times higher for sub-60 years old unvaccinated individuals than CoronaVac vaccines (RR = 5.4, p = 0.044). While the risk of death was also higher for the same group (RR = 2.5, p = 0.036) (Table 2).
Laboratory tests results showed that reactive C protein was increased in every recruited patient, vaccinated or not, displaying only un-significant differences (Kruskal Wallis, p = 0.765) (Table 3). Notably, CanSino and BNT162b2-vaccines patients presented lower levels of D dimer than those vaccinated with CoronaVac, incomplete schemes, or unvaccinated individuals. In addition, every CanSino and BNT162b2 vaccine displayed a lymphocyte count lower than reference threshold (< 1,000 pL). Furthermore, 72.1% of CoronaVac vaccinees and 72.7% of unvaccinated individuals showed similarly low lymphocyte counts. Complementarily, 2.33% of CoronaVac vaccinees, and 3.64% of unvaccinated individuals showed a lymphocyte count over reference threshold (> 4800/pL).
These observations of hospitalized COVID-19 patients were complemented with identified SARS-CoV-2 variants for 77 cases (48.4%). In this sense, only 1 individual was identified to be infected by the Lambda variant (1.3%), 2 by the Mu variant (2.6%), 24 by the Delta variant (31.17%), and 50 by the Gamma variant (64.94%) (Figure 1). Interestingly, when symptom frequencies were summarized according to Delta or Gamma variant infection, fever, cough, and myalgia were more frequent across Gamma-infected individuals. In contrast, dyspnea and anosmia were more frequently reported by Delta-infected individuals. Among Delta-infected individuals, cough, myalgia, dyspnea, and anosmia symptoms were more frequently reported by unvaccinated individuals than by CoronaVac vaccines. Interestingly, fever was more frequently reported by Gamma-infected CoronaVac vaccines than by Gamma-infected unvaccinated individuals, in opposition to the frequency observed for cough, myalgia, dyspnea, and anosmia symptoms (Figure 1 and 2).
-
Discussion
First, it is important to note that every individual recruited by our study suffered a severe COVID-19 condition and that the proportion of vaccinated and unvaccinated individuals is comparable. In this sense, it resembles a picture of the occupation status of CPU southern Chile, when notwithstanding a massive vaccination program was being applied, health services were challenged. In this sense, the group of CoronaVac vaccinated patients displayed a strong enrichment of Arterial Hypertension and Diabetes Mellitus in respect to unvaccinated individuals, which is in hand with the priority they had to receive first vaccine shots available[7]. On the other hand, during this time window, we found that the sub-set of recruited patients older than 60 years old were predominantly unvaccinated, whilst they also had a higher risk of death, same as extensively reported[26],[27]. In contrast, when presenting severe COVID-19, vaccinated individuals younger than 60 years old do not have a different risk of death than unvaccinated, suggesting that once suffering from severe COVID-19, previous vaccineinduced immunity is not sufficient to protect them from death. These results coincide with the largest study carried out in Chile on the effectiveness of the CoronaVac vaccine, which determined that it especially conferred greater protection to those over 60 years of age for mortality from COVID-19[24]. Likewise, a study carried out in Brazil for seriously ill or deceased patients with COVID-19 highlights mortality (for patients vaccinated with CoronaVac) of younger age, but who were health workers[28].
Table 1. Characterization of demographics, symptoms, comorbidities, and complications of COVID-19 patients, according to their vaccination status
Table 2: Risk factors according to age and complications for different age and complications subgroups
Furthermore, the critical contribution of vaccine platforms other than CoronaVac to the challenging circumstances of mid-2021, is evident. For instance, although the group of CoronaVac vaccinated (n = 63) did not have a lower risk of requiring oxygen support than unvaccinated individuals, the incorporation of only 6 BNT162b2 and 6 CanSino-vaccines to the analysis allowed to a comparatively and significantly decrease this risk across vaccinated patients.
Also supporting a differential clinical progression between the individuals of our cohort vaccinated with different formulations, laboratory parameters display different mean values between those groups. The most dissimilar parameter analyzed is D-Dimer concentration, for which CoronaVac vaccines show mean levels more than 160 times higher than BNT162b2 or CanSino-vaccinated individuals. D-Dimer is a well-known predictor of COVID-19 severity and mortality[29], so its elevated levels are expected among severe individuals like every patient from our cohort, but notably, CoronaVac vaccines displayed mean levels 1.25 times higher than individuals without any vaccine shot.
Our results also suggest that, despite the evolved nature of SARS-CoV-2 variants in respect to the Wuhan reference genome used in CoronaVac inactivated vaccine formulation, complete vaccination scheme confer protection from death to the elderly.
-
Limitations of the study
Although this work displays a detailed picture of the vaccination status of severely hospitalized individuals in southern Chile, it is a descriptive study that could have been more informative if more clinical parameters were captured. Nevertheless, its cohort size and balanced composition allowed for obtaining critical information on the vaccine’s effects on the most affected individuals during one of the deadliest stages of the COVID-19 pandemic.
-
Conclusions
Hospitalized patients with COVID-19 in the present study more commonly presented hypertension and Diabetes Mellitus. Patients vaccinated with CoronaVac presented cough, dyspnea, and the need for invasive mechanical ventilation less frequently than unvaccinated patients. The risk of death was lower for people vaccinated with CoronaVac than for those not vaccinated, but only for people older than 60 years, while the risk of needing intensive mechanical ventilation or oxygen was significantly lower only for people vaccinated with Coro- naVac younger 60 years old.
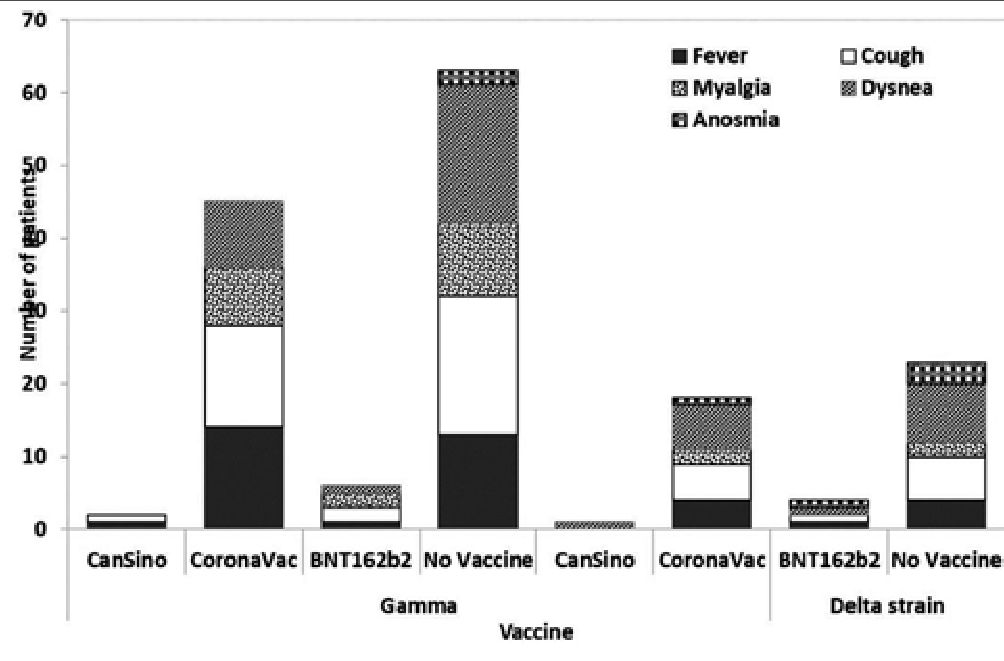
Figure 1. Reported symptoms of COVID-19 individuals infected with an identified SARS-CoV-2 variant of concern.
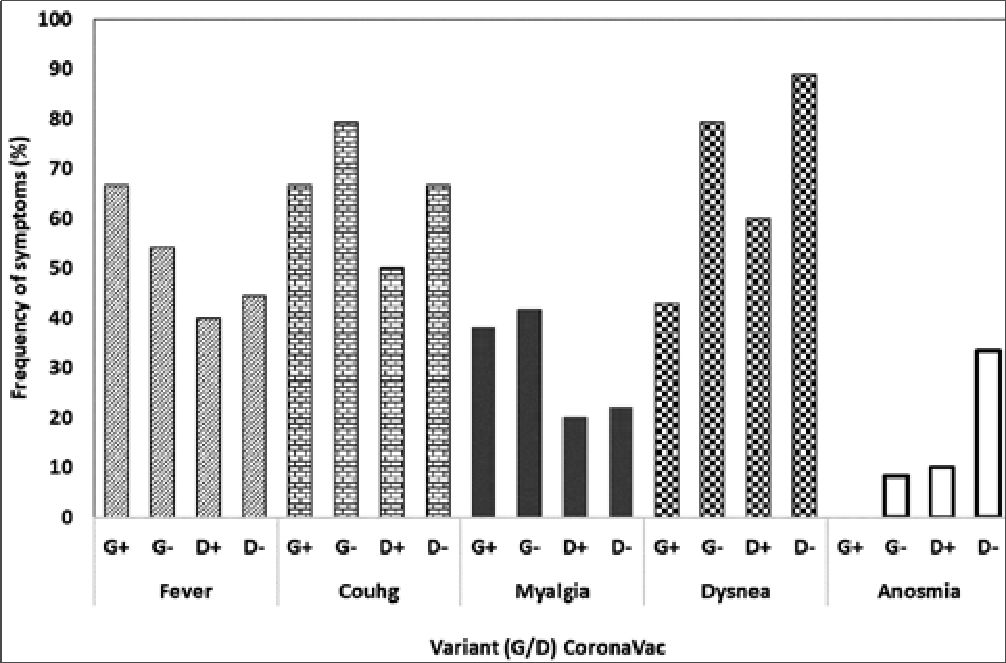
Figure 2. Symptom frequency according to SARS-CoV-2 variant.
Table 3. Laboratory tests results of COVID-19 patients, according to their vaccination status
| Statistics | CanSino | BNT162b2 | CoronaVac | Incomplete dose | No vaccine | P |
| C Reactive Protein (PCR) (mg/l) | ||||||
| [0.00 – 0.50] mg/dL | ||||||
| n | 5 | 5 | 49 | 7 | 62 | |
| Median | 163.0 | 107.0 | 145.5 | 102.0 | 116.2 | 0.765 |
| % Over range | 100.0 | 80.0 | 98.0 | 100.0 | 100.0 | |
| First quartile | 136.0 | 6.1 | 71.1 | 89.4 | 54.6 | |
| Third quartile | 190.2 | 202.1 | 200.0 | 191.7 | 201.3 | |
| interquartile range | 54.2 | 196.0 | 128.9 | 102.3 | 146.7 | |
| Min. | 94.0 | 0.3 | 0.4 | 75.1 | 0.6 | |
| Max. | 238.8 | 252.2 | 3493.0 | 363.0 | 821.0 | |
| D Dimer (pg/mL) | ||||||
| < 0.5 micrograms per milliliter | ||||||
| n | 4 | 5 | 30 | 5 | 48 | |
| Median | 0.9 | 0.8 | 145.5 | 102.0 | 116.2 | 0.655 |
| % Under range | 25.0 | 20.0 | 23.3 | 40.0 | 37.5 | |
| First quartile | 0.6 | 45.8 | 71.1 | 89.4 | 54.6 | |
| Third quartile | 1.0 | 143.5 | 200.0 | 191.7 | 201.3 | |
| interquartile range | 0.4 | 97.7 | 128.9 | 102.3 | 146.7 | |
| Min. | 0.2 | 0.2 | 0.4 | 75.1 | 0.6 | |
| Max. | 1.1 | 3493.0 | 3493.0 | 363.0 | 821.0 | |
| Lymphocytes | ||||||
| 1,000 – 4,800 (pL) | ||||||
| n | 5 | 5 | 43 | 6 | 55 | |
| Median | 405.0 | 506.0 | 770.0 | 729.0 | 672.0 | 0.361 |
| % Under 1.000 | 100.0 | 100.0 | 72.1 | 50.0 | 72.7 | |
| % Over 4.800 | 0.00 | 0.00 | 2.33 | 0.00 | 3.64 | |
| First quartile | 1.4 | 4.7 | 7.7 | 97.9 | 29.5 | |
| Third quartile | 451.0 | 630.0 | 1032.5 | 1113.8 | 1005.5 | |
| interquartile range | 449.6 | 625.3 | 1024.8 | 1015.9 | 976.0 | |
| Min. | 0.4 | 2.4 | 0.4 | 0.5 | 0.4 | |
| Max. | 759.0 | 631.0 | 26900.0 | 1641.0 | 9100.0 |
It is necessary to identify worldwide the effects of the different vaccines by age and their follow-up over the next few years. Future studies can help countries in decision-making in vaccination schemes for what will be the endemic of COVID-19
Acknowledgments: We thank Dr. Maria Inés Barría for her support and medical interns of the Hospital de Castro: Claudio Aburto Luer, Bruno Alonso Merino Nievas and Michelle Alexandra Berger Rivera for their support in data collection. We also thank:
• María Luisa Rioseco Zorn, Hospital Puerto Montt Dr. Eduardo Schütz Schroeder, Puerto Montt, Chile.
• María Alejandra Lobos Floody, Hospital de Castro Dr. Augusto Riffart, Castro, Chile.
• Mario Barra Zapata, Hospital de Castro Dr. Augusto Riffart, Castro, Chile.
• Stephania Astried Passalacqua Hidalgo, Hospital Base San José Osorno. Osorno, Chile.
• Carla Marcela Mayorga Hernández, Hospital Base San José Osorno. Osorno, Chile.
• Carmen Gloria Zamorano Vásquez, Hospital Base San José Osorno. Osorno, Chile.
• Marcos Gilbert Godoy Gatica, Faculty of Veterinary Medicine Universidad San Sebastián. Puerto Montt, Chile. Centro de Investigaciones Biológicas Aplicadas (CIBA). Puerto Montt, Chile.
• Marleny Marin Catalán, Internal Medicine Training Program, Faculty of Medicine and Science. Universidad San Sebastian. Puerto Montt. Chile.
• Claudia Patricia Gómez Ocampo, Internal Medicine Training Program, Faculty of Medicine and Science. Universidad San Sebastian. Puerto Montt, Chile.
• Nicolás Atuesta Dimian, Geriatric Training Program. Faculty of Medicine and Science, Universidad San Sebastian. Puerto Montt, Chile.
• Michelle Alexandra Berger Rivera, Internship (medicine) Universidad San Sebastian. Puerto Montt, Chile.
• Bruno Merino Nievas, Internship (medicine) Universidad San Sebastian. Puerto Montt, Chile.
• Claudio Aburto Luer, Internship (medicine) Universidad San Sebastian. Puerto Montt, Chile.
Conflicts of interest: The authors declare no conflict of interest.
Funding: This research received no external funding.
Fecha de recepción: 04 de abril de 2023 / Fecha de aceptación: 16 de mayo de 2023
References
1. Organization WH. Available from: https://covid19.who.int
2. Fund UN. Available from: https://www.unfpa.org/data/world-population-dashboard
3. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol (2021) 21(10):626-36. Epub 20210809. https://doi.org/10.1038/s41577-021-00592-1 ..
4. Muena NA, Garcia-Salum T, Pardo-Roa C, Serrano EF, Levican J, Avendano MJ, et al. Long-lasting neutralizing antibody responses in SARS-CoV-2 seropositive individuals are robustly boosted by immunization with the CoronaVac and BNT162b2 vaccines. medRxiv (2021). Epub 20210518. https://doi.org/10.1101/2021.05.17.21257197.
5. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al.; CoronaVac Study Group. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021 Jul;398(10296):213–22. https://doi.org/10.1016/S0140-6736(21)01429-X PMID:34246358
6. Reyes H, Diethelm-Varela B, Méndez C, Rebolledo-Zelada D, Lillo-Dapremont B, Muñoz SR, et al. Contribution of Two-Dose Vaccination Toward the Reduction of COVID-19 Cases, ICU Hospitalizations and Deaths in Chile Assessed Through Explanatory Generalized Additive Models for Location, Scale, and Shape. Front Public Health. 2022 Jul;10:815036. https://doi.org/10.3389/fpubh.2022.815036 PMID:35968462
7. Chile MdSdlRd. Available from: https://www.minsal.cl/nuevo-coronavirus-2019-ncov/informe-epidemiologico-covid-19/
8. Randolph HE, Barreiro LB. Herd Immunity: understanding COVID-19. Immunity. 2020 May;52(5):737–41. https://doi.org/10.1016/j.immuni.2020.04.012 PMID:32433946
9. Mistry P, Barmania F, Mellet J, Peta K, Strydom A, Viljoen IM, et al. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front Immunol (2021) 12:809244. Epub 20220103. https://doi.org/10.3389/fimmu.2021.809244..
10. Bloom JD. Recovery of Deleted Deep Sequencing Data Sheds More Light on the Early Wuhan SARS-CoV-2 Epidemic. Mol Biol Evol. 2021 Dec;38(12):5211–24. https://doi.org/10.1093/molbev/msab246 PMID:34398234
11. Thakur N, Das S, Kumar S, Maurya VK, Dhama K, Paweska JT, et al. Tracing the origin of Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A systematic review and narrative synthesis. J Med Virol (2022) 94(12):5766-79. Epub 20220907. https://doi.org/10.1002/jmv.28060..
12. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Netw Open (2020) 3(12):e2029058. Epub 20201201. https://doi.org/10.1001/jamanetworkopen.2020.29058..
13. Boehm E, Kronig I, Neher RA, Eckerle I, Vetter P, Kaiser L, et al. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect (2021) 27(8):1109-17. Epub 20210517. https://doi.org/10.1016/j.cmi.2021.05.022..
14. Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med (2022) 20(1):200. Epub 20220523. https://doi.org/10.1186/s12916-022-02397-y..
15. Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature (2021) 593(7858):266-9. Epub 20210325. https://doi.org/10.1038/s41586-021-03470-x ..
16. Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature (2021) 592(7854):438-43. Epub 20210309. https://doi.org/10.1038/s41586-021-03402-9..
17. Fujino T, Nomoto H, Kutsuna S, Ujiie M, Suzuki T, Sato R, et al. Novel SARS-CoV-2 Variant in Travelers from Brazil to Japan. Emerg Infect Dis (2021) 27(4). Epub 20210210. https://doi.org/10.3201/eid2704.210138..
18. Ferreira IA, Kemp SA, Datir R, Saito A, Meng B, Rakshit P, et al.; CITIID-NIHR BioResource COVID-19 Collaboration, Indian SARS-CoV-2 Genomics Consortium; Genotype to Phenotype Japan (G2P-Japan) Consortium. SARS-CoV-2 B.1.617 Mutations L452R and E484Q Are Not Synergistic for Antibody Evasion. J Infect Dis. 2021 Sep;224(6):989–94. https://doi.org/10.1093/infdis/jiab368 PMID:34260717
19. Zhang W, Davis BD, Chen SS, Sincuir Martinez JM, Plummer JT, Vail E. Emergence of a Novel SARS-CoV-2 Variant in Southern California. JAMA. 2021 Apr;325(13):1324–6. https://doi.org/10.1001/jama.2021.1612 PMID:33571356
20. Laiton-Donato K, Franco-Munoz C, Alvarez-Diaz DA, Ruiz-Moreno HA, Usme-Ciro JA, Prada DA, et al. Characterization of the emerging B.1.621 variant of interest of SARS-CoV-2. Infect Genet Evol (2021) 95:105038. Epub 20210814. https://doi.org/10.1016/j.meegid.2021.105038..
21. Coronaviridae Study Group of the International Committee on Taxonomy of V. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol (2020) 5(4):536-44. Epub 20200302. https://doi.org/10.1038/s41564-020-0695-z..
22. Abdool Karim SS, de Oliveira T. New SARS-CoV-2 Variants – Clinical, Public Health, and Vaccine Implications. N Engl J Med (2021) 384(19):1866-8. Epub 20210324. https://doi.org/10.1056/NEJMc2100362..
23. Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell (2021) 184(9):2372-83 e9. Epub 20210312. https://doi.org/10.1016/j.cell.2021.03.013..
24. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. 2021 Sep;385(10):875–84. https://doi.org/10.1056/NEJMoa2107715 PMID:34233097
25. Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022 Jan;114:252–60. https://doi.org/10.1016/j.ijid.2021.11.009 PMID:34800687
26. Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS, et al. Predictors of COVID-19 severity: A literature review. Rev Med Virol (2021) 31(1):1-10. Epub 20200730. https://doi.org/10.1002/rmv.2146..
27. Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, et al. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res Rev (2021) 65:101205. Epub 20201031. https://doi.org/10.1016/j.arr.2020.101205..
28. Campos KR, Sacchi CT, Abbud A, Caterino-de-Araujo A. SARS-CoV-2 variants in severely symptomatic and deceased persons who had been vaccinated against COVID-19 in São Paulo, Brazil. Rev Panam Salud Publica. 2021 Oct;45:e126. https://doi.org/10.26633/RPSP.2021.126 PMID:34707647
29. Shah S, Shah K, Patel SB, Patel FS, Osman M, Velagapudi P, et al. Elevated D-Dimer Levels Are Associated With Increased Risk of Mortality in Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Cardiol Rev (2020) 28(6):295-302. Epub 20200702. https://doi.org/10.1097/CRD.0000000000000330..

 ORCID
ORCID

 Creative Commons Attribution
Creative Commons Attribution