Noha Afify MD.1,*, Khaled Gaballah MD.1
Recibido: 03-04-2023
Aceptado: 01-08-2023
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 6 pp. 604-610|https://doi.org/10.25237/revchilanestv52n6-08
PDF|ePub|RIS
Eficacia analgésica de la nalbufina subcutánea comparada con analgesia epidural en cirugías abdominales: un ensayo controlado aleatorio
Abstract
Background: Analgesia represents a cornerstone for the perioperative management of patients undergoing open abdominal surgery. Epidural analgesia (EPA) is a standard method for pain control in 50%-60% of major abdominal surgeries. Nalbuphine -a synthetic opioid agonist/ antagonist- has equianalgesic properties to morphine but without the undesirable side effects of the pure agonists. Objective: We compared the perioperative analgesic efficacy of subcutaneous nalbuphine and epidural analgesia in abdominal surgeries. Methods: 130 adult patients of both sexes with American Society of Anesthesiologists physical status class I or II aged 20-60 years were randomized into two equal groups. After induction of general anesthesia, the epidural was activated with 18 ml of bupivacaine 0.25% + 2 ml fentanyl (100 Mcg) in group (I), while in a group (II) subcutaneous nalbuphine (0.15 mg/kg) was injected. The time for the first postoperative analgesic request was recorded as the primary endpoint. The cumulative analgesics consumption within the first 24 postoperative hours and drug-related side effects were the secondary outcomes. Results: The time for the first postoperative analgesia request was significantly longer in group II than in group I (10.8 ± 1.34 vs 3.29 ± 0.72 hours) (p < 0.05). There was a significant reduction in postoperative analgesic requirements in group II compared with group I. Group II patients had significantly more sedation levels than group I (p < 0.05). Conclusion: Under the conditions of this study, subcutaneous nalbuphine showed a superior analgesic profile than epidural analgesia for open abdominal surgeries.
Resumen
Antecedentes: La analgesia representa un pilar en el manejo perioperatorio de los pacientes sometidos a cirugía abdominal abierta. La analgesia epidural (EPA) es un método reconocido para controlar el dolor en el 50%-60% de las cirugías abdominales mayores. La nalbufina -un ago- nista/antagonista opioide sintético- tiene propiedades equianalgésicas a la morfina pero sin los efectos secundarios indeseables de los agonistas puros. Objetivo: Comparamos las propiedades analgésicas perioperatorias de la nalbufina subcutánea y la analgesia epidural en cirugías abdominales. Métodos: 130 pacientes adultos de ambos sexos ASA I o II, entre 20 a 60 años de edad fueron aleatorizados en dos grupos. Después de la inducción de la anestesia general y antes de la incisión en la piel, se activó la epidural con 18 ml de bupivacaína al 0,25% + 2 ml de fentanilo (100 Mcg) en el grupo (I), mientras que en el grupo (II) se inyectó nalbufina subcutánea (0,15 mg/kg). El momento de la primera solicitud de analgésicos posoperatorios se registró como el objetivo primario. Los resultados secundarios fueron el consumo acumulado de analgésicos dentro de las primeras 24 h posoperatorias y los efectos secundarios relacionados con los fármacos. Resultados: El tiempo para la primera solicitud de analgesia posoperatoria fue significativamente mayor en el grupo II que en el grupo I (10,8 ± 1,34 vs 3,29 ± 0,72 h) (p < 0,05). Hubo una reducción significativa en los requerimientos analgésicos posoperatorios en el grupo II en comparación con el grupo I. Los pacientes del grupo II tenían significativamente más niveles de sedación que el grupo I (p < 0,05). Conclusión: En las condiciones de este estudio, la nalbufina subcutánea mostró un perfil analgésico superior a la analgesia epidural para cirugías abdominales abiertas.
-
Introduction
Analgesia represents a cornerstone for the perioperative management of patients undergoing open abdominal surgery[1]. Epidural analgesia (EPA) is an effective pain control modality that is used in approximately 50%-60% of major abdominal surgeries[2].
Epidural analgesic modality has become limited to special conditions, in which pain management is difficult. So other analgesic methods should be available[3].
Nalbuphine is a derivative of 14-hydroxy morphine with kappa receptor agonistic and partial mu receptor antagonistic effects. It can safely and effectively provide equianalgesic effects to morphine in visceral pain modulation with a ceiling effect on respiratory depression and less nausea, vomiting, and pruritus[4]. It is used effectively to treat moderate to severe pain for a slightly longer duration than morphine. Subcutaneous drug administration has many significant advantages; it allows efficient drug absorption and stable blood serum drug concentration in addition to using small volumes for a long time[5].
The aim of this randomized controlled study was to compare the effect of subcutaneous nalbuphine and epidural analgesia on postoperative first call for analgesics and opioid consumption. We hypothesized that subcutaneous nalbuphine may be a good alternative to epidural analgesia when epidural is contraindicated or failed to be inserted in patients undergoing open abdominal surgeries.
-
Methods
This prospective single-blinded RCT was conducted at Menoufia University hospitals between December 2019 and March 2020. The trial was registered at www.pactr.org (PAC- TR2020001875909224). The approval of the local ethics and research committee of the faculty of medicine of Menoufia University was obtained in December 2019 (IRB approval number 12-2019 ANET 52A). Written informed consent was collected from all eligible patients. The present report of this RCT runs in concordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
We studied 130 adult patients (20-60 years of age) of both sexes with American Society of Anesthesiologists physical status class I or II undergoing elective open abdominal surgeries (expected not to exceed three hours) under general anesthesia.
Patients on opioid therapy, central nervous system depressants or with physical dependence on opioids, hepatic or renal disease, pregnancy or lactation, diabetes mellitus, bronchial asthma, cardiac disease, and/or bleeding disorder were not included. We also excluded patients refusing or having a contraindication for neuraxial blocks, neurological dysfunction, established respiratory depression; a history of hypersensitivity to any of the study drugs, inability to understand the visual analog scale (VAS).
All patients meeting the inclusion criteria without exclusion criteria were randomized 1:1 using a computerized software program. The allocation was masked for the trial investigators and the trial statistician who conducted the analyses. Before the surgery, the visual analog scale (VAS) was taught to the included patients to express and quantify their pain.
All patients were premedicated with bromazepam (1.5 mg) the night before surgery and 2 hours before the call to the operative theatre. When the patients arrived at the operating room, continuous electrocardiography, non-invasive blood pressure, pulse oximetry, train of four (TOF) guard, and bispectral index (BIS) monitors were applied. An 18-gauge cannula was inserted in a peripheral vein, and infusion of (7 ml/kg/h) of lactated ringer was started. Preanesthetic medications included intravenous (IV) glycopyrrolate (0.04 mg/kg) and midazolam (0.03 mg/kg).
In group (I): in a sitting position, an epidural catheter was inserted before anesthetic induction under the complete aseptic condition at the vertebral level expected to be the central dermatome of the planned surgical site. A local anesthetic was injected into the skin, then an 18 G Tuohy needle was introduced. The epidural space was identified using the loss of resistance to air technique, and a multi-orifice 20 G epidural catheter (Perifix® complete set; B. Braun Melsungen AG, Melsungen, Germany) was placed 3-4 cm within the epidural space. After the removal of the needle, A test dose was injected, and the proper epidural position was confirmed.
Anesthesia was induced with propofol (2 mg/kg), fentanyl (2 pg/kg), and atracurium (0.5 mg/kg). After oral endotracheal intubation, anesthesia was maintained with isoflurane (1%- 2%) on an O2/air mixture (FiO2 0.5) and atracurium (0.25 mg/ kg) guided by the TOF guard. The patients were mechanically ventilated targeting ETCO2 of 35-40 mmHg. Isoflurane MAC was adapted to keep BIS 40-50.
After induction, the epidural was activated with bupiva- caine 0.25% (18 ml) + fentanyl (100 Mcg) (2 ml) in group (I), while in group (II) nalbuphine (0.15 mg/kg) (maximally 20 mg) was injected SC in the outer aspect of the upper arm using 25 gauge needle. Dexamethasone (8 mg) and granisetron (1 mg) were given IV immediately after induction as prophylactic antiemetics.
If analgesia was required, indicated by hemodynamic values rising above the targeted figures (20% above the baseline value) in presence of accepted BIS, ETCO2, SpO2, and muscle relaxation, 6 ml of the epidural solution was given in group I and 0.05 mg/kg SC nalbuphine was injected in group II. Rescue analgesia was given intraoperatively as acetaminophen (15 mg/ kg) IV over 10 minutes if the top-up dose was deemed insufficient. Hypotension (mean arterial blood pressure less than 60 mmHg or < 80% of the baseline value) was managed by incremental doses of ephedrine (5 mg).
After surgery completion, residual neuromuscular blockade was antagonized with neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg). All patients were extubated and transferred to the post-anesthesia care unit. Acetaminophen (1 gm) was infused every 6 hours on the first postoperative day.
Postoperatively, the pain was assessed using the VAS (0-10, with 0 as no pain and 10 as the maximum possible pain). VAS was assessed 30 min after extubation (0), then at 3,6, 9, 12, 18, and 24 hours. If VAS was > four, ketorolac 30 mg was slowly infused. After 10 minutes, if VAS remained > four, nalbuphine (0.15mg /kg) was given IV.
Efficacy measures:
The primary outcome was the time for the first postoperative analgesic request after recovery. The secondary outcome
measures were the VAS, the cumulative analgesics consumption in the first 24 postoperative hours, the intraoperative analgesics needs, complications (nausea and vomiting, respiratory depression, pruritus), and patient satisfaction using satisfaction score (nil = 0; mild = 1; good = 2; excellent = 3).
Postoperative sedation was assessed at times 0, 10, 20, and 30 min after extubation using the Ramsay sedation scale (RSS) (1, anxious or restless or both. 2, cooperative, orientated and tranquil. 3, responding to commands. 4, brisk response to a stimulus. 5, sluggish response to stimulus. 6, no response to stimulus)[6].
Postoperative nausea and vomiting (1: no nausea, 2: mild nausea, 3: severe nausea, 4: vomiting), respiratory depression (respiratory rate < 8/min or SpO2< 90%), hypotension (systolic blood pressure < 60 mmHg) and bradycardia (pulse rate < 60/ min) were recorded.
-
Sample analysis and statistical analysis
The sample size was calculated by the Epi info program based on a previous study[7], in which there was a significant difference between the thoracic epidural analgesia and rectus sheath analgesia in the first request for morphine (p = 0.031). To achieve a power of 80% to detect this difference with a significance level of 5% and a confidence interval of 95%, it was estimated that 130 subjects were required in the study divided into two equal groups.
After data collection and tabulation, it statistically analyzed using an IBM personal computer with Statistical Package of Social Science (SPSS) version 22 (SPSS, Inc, Chicago, Illinois, USA).
Data were presented as descriptive statistics in which quantitative data were presented in the form of the mean (X), standard deviation (SD), range, and qualitative data were presented in the form of numbers and percentages and analytical statistics in which the used tests of significance included Chisquare test (x2) for two qualitative variables and Student t-test for two quantitative variables. We used the Mann-Whitney test for comparison between two groups not normally distributed having quantitative variables.
-
Results
One hundred thirty patients were included in the study without dropouts (Figure 1). There were no significant differences between the study groups regarding demographic characteristics and/or surgery type and duration (Table 1).
Group II had a significantly longer time for first postoperative analgesia than group I (10.8 ± 1.34 and 3.29 ± 0.72 hours) respectively (p < 0.001). The cumulative postoperative analgesic requirements were significantly lower in group II in comparison to the group I (p < 0.001). The intraoperative analgesic consumption was statistically comparable between both groups (p = 0.143). Intraoperative rescue analgesia was needed in four patients in group II in comparison to nine patients in group I (p = 0.143) (Table 2).
During the first 4 postoperative hours, the VAS was significantly lower in group II (p < 0.001). However, during the 8-12th postoperative hours, the VAS was significantly lower in group I (p <0.001). Both groups had comparable VAS at the remaining
time points (Figure 2).
The postoperative sedation score was significantly lower in group I for 30 min (p < 0.001) and no other complications could be detected in both group. As regards patient satisfaction, excellent, good, and mild satisfaction scores were recorded at (80% vs 0%, 20% vs 83%, and 0% vs 17%) (p < 0.001) in groups II and I respectively (Table 3).
-
Discussion
This study showed that subcutaneous nalbuphine showed a superior postoperative analgesic profile than epidural analgesia for open abdominal surgeries. Both analgesia modalities were associated with a comparable intraoperative analgesia profile.
Nalbuphine is known to provide a reasonably potent analgesia which is comparable to that of morphine in visceral nociception as it has both agonistic action at kappa and antagonist activity at p opioid receptors. It improves the quality of postoperative analgesia with fewer side effects[8]. It also maintains or even enhances p-opioid-based analgesia and blocks the central sensitization caused by surgical trauma or nociceptive stimulation[9],[10]. Finally, its analgesic and sedative effects are improved by increasing opioid receptor density and activity[11]. Nalbuphine was studied in detail as an intramuscular or IV analgesic or as an adjuvant to local anesthetics in epidural and intrathecal anesthesia for abdominal surgeries[12]-[14].
The SC opioid injection is an established and as effective as IV injection also, it is a safe and inexpensive analgesic method with minimal side effects. The efficacy of SC opioid delivery for controlling pain was studied in a range of patient populations, including postsurgical and cancer patients[15],[16].
In our study, SC nalbuphine significantly prolonged the time for the first postoperative analgesic request and reduced the postoperative analgesic requirements within the first 24 hours than did the epidural analgesia.
These results are consistent with other studies that investigated the analgesic efficacy of nalbuphine. Solanki and his col- leagues[17], injected the same dose we used IV in patients undergoing orthopedic surgeries. They reported that nalbuphine did not only effectively alleviates moderate to severe postoperative pain, but also provided good sedation and hemodynamic stability with a lower incidence of nausea and vomiting in comparison to tramadol. In comparison with tramadol[18], fentanyl[19], or pentazocine[20], nalbuphine was associated with a better relief of postoperative pain in conjunction with more favorable sedation, hemodynamic stability, and lower incidence of PONV in short surgical procedures.
In their meta-analysis of 15 trials (820 patients) to compare nalbuphine with morphine regarding the safety profile and quality of analgesia, Zeng and his colleagues[4] reported a comparable analgesic potency with a superior safety profile for nalbuphine due to the absence of respiratory depressant effects and lower incidence of other side effects, which were observed with morphine.
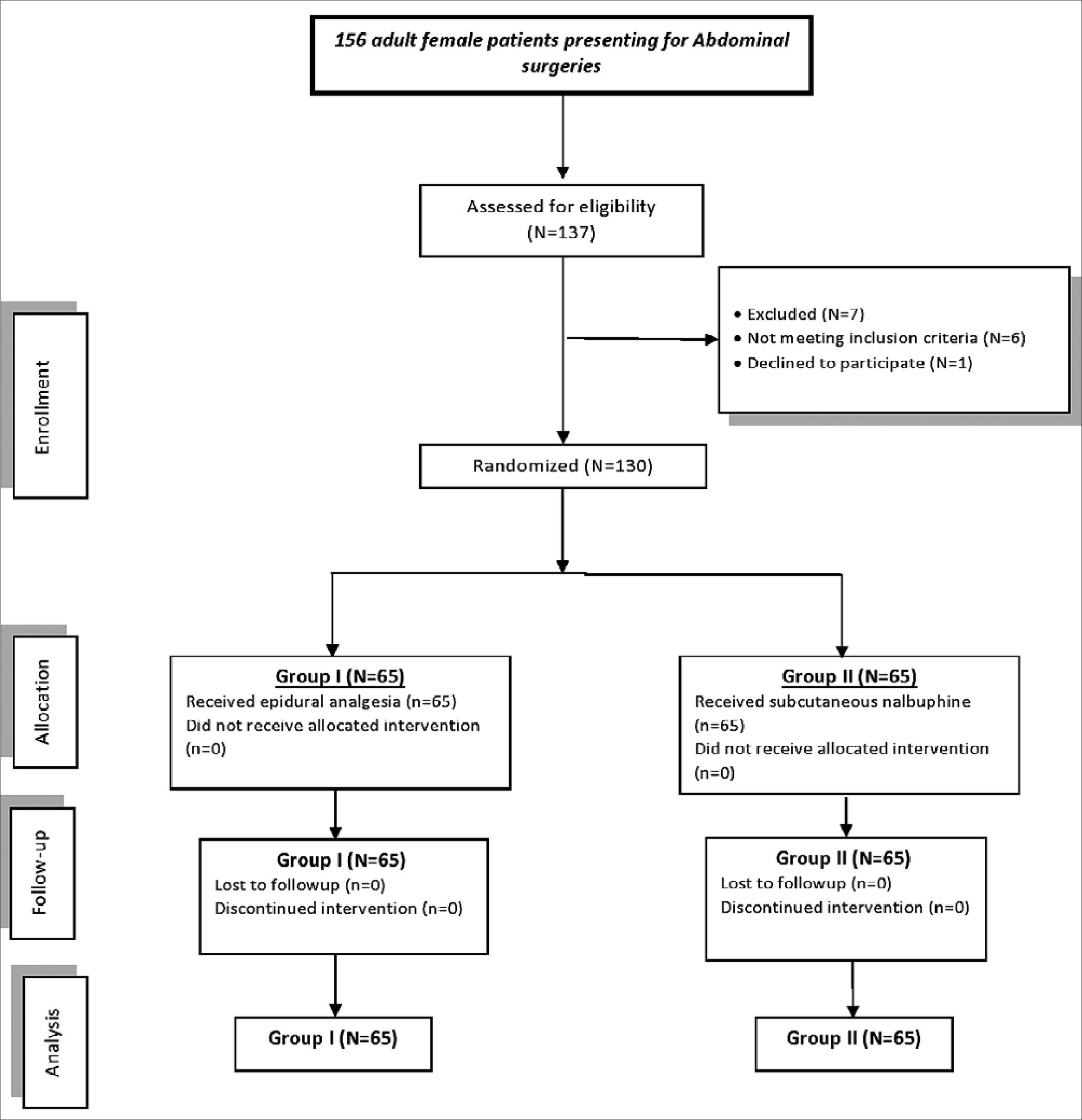
Figure 1. Study flowchart.
Epidural analgesia may provide satisfactory analgesia that allows early mobilization. However, it may be difficult to mobilize the patient with the epidural pump or in presence of even a mild degree of motor block, which may consequently delay being out of the bed. Furthermore, the epidural route may not be of choice for those undergoing laparoscopic procedures with a short hospital stay. Moreover, epidural analgesia did not add clinical benefits after laparoscopic colorectal surgery[21]. Furthermore, it was not protective against prolonged opioid use after abdominal surgery[22]. Unlike epidural analgesia, SC nalbuphine injection does not need a skilled anesthetist or necessitate close monitoring for side effects e.g, hypotension, and motor block[23]. Reducing the need for supplementary analgesics in the immediate postoperative period may result in cost savings that have financial implications for developing countries and low-resource settings.
In presence of a catheter, the epidural route may be a very effective analgesia modality for post-laparotomy pain during both rest and ambulation. However, based on this study’s findings, SC nalbuphine may be a good alternative to epidural analgesia for abdominal surgeries in low resources settings and/ or when an epidural is contraindicated or failed to be inserted. Furthermore, unlike epidural, SC nalbuphine will not mandate admitting the patient to a high-dependency unit for follow-up. Further research is needed to determine the appropriate dose of SC nalbuphine to achieve the target analgesic need and compare it with other analgesic modalities.
Table 1. Demographic and clinical data of the studied groups
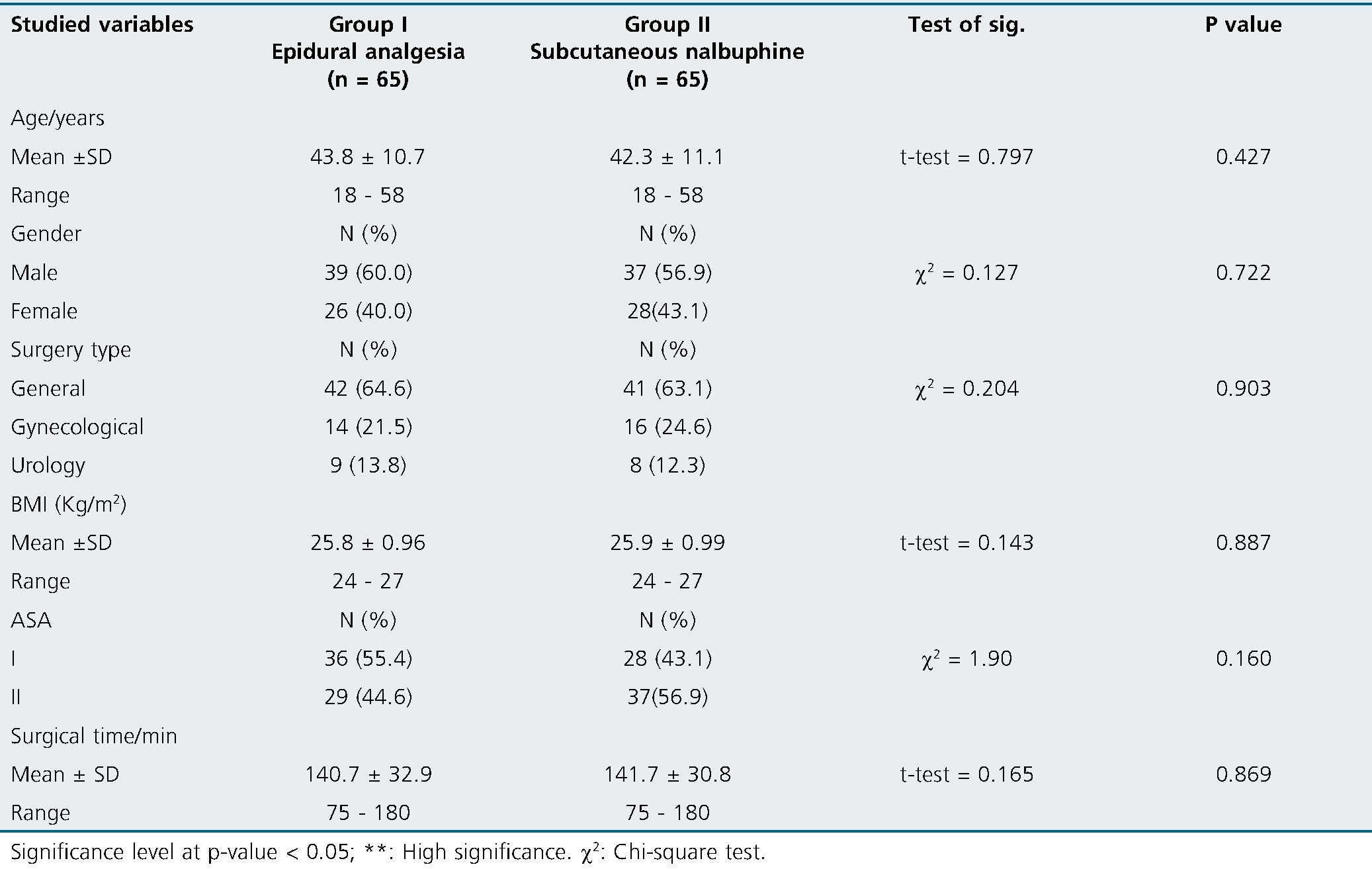
Table 2. Analgesic requirements among the studied groups
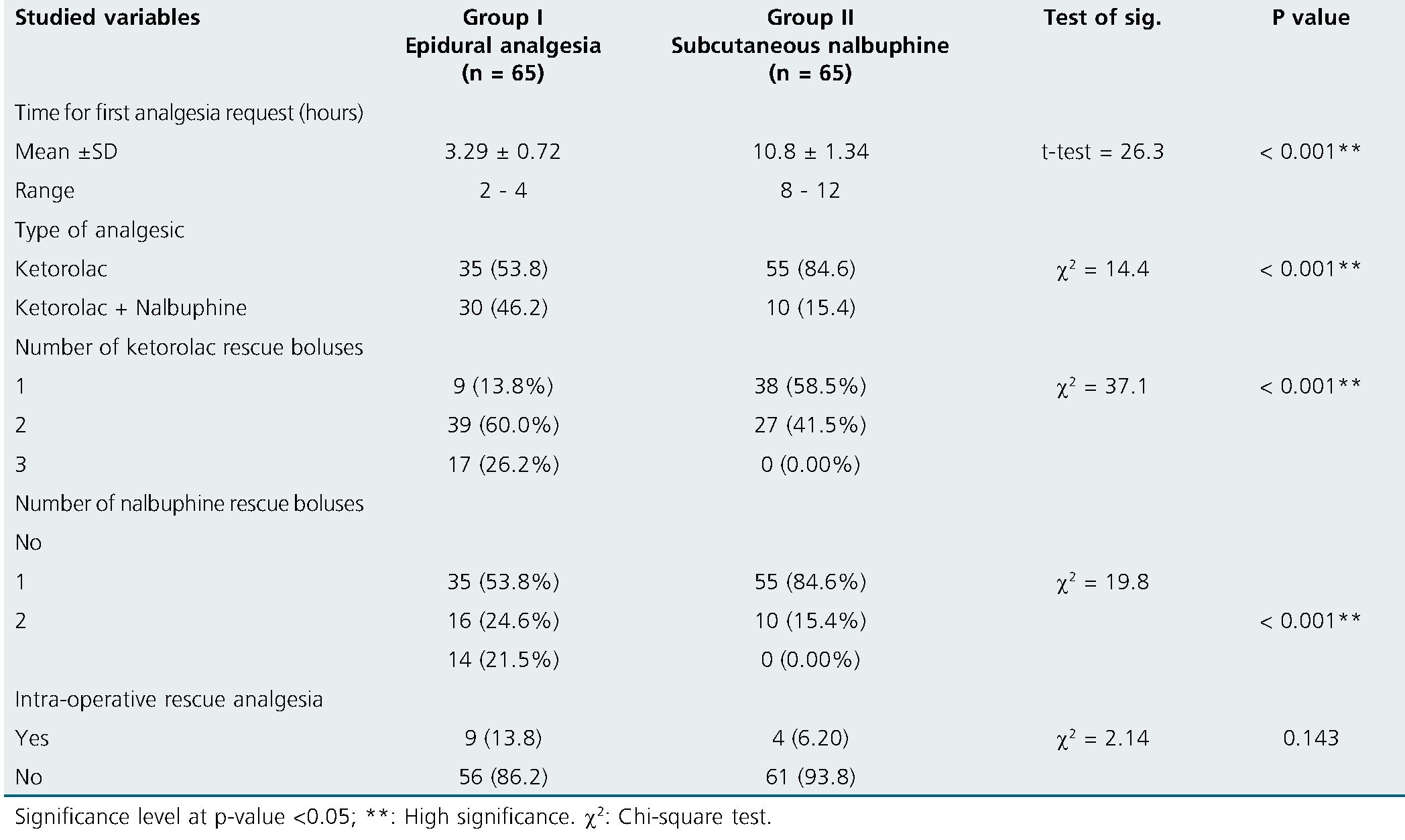
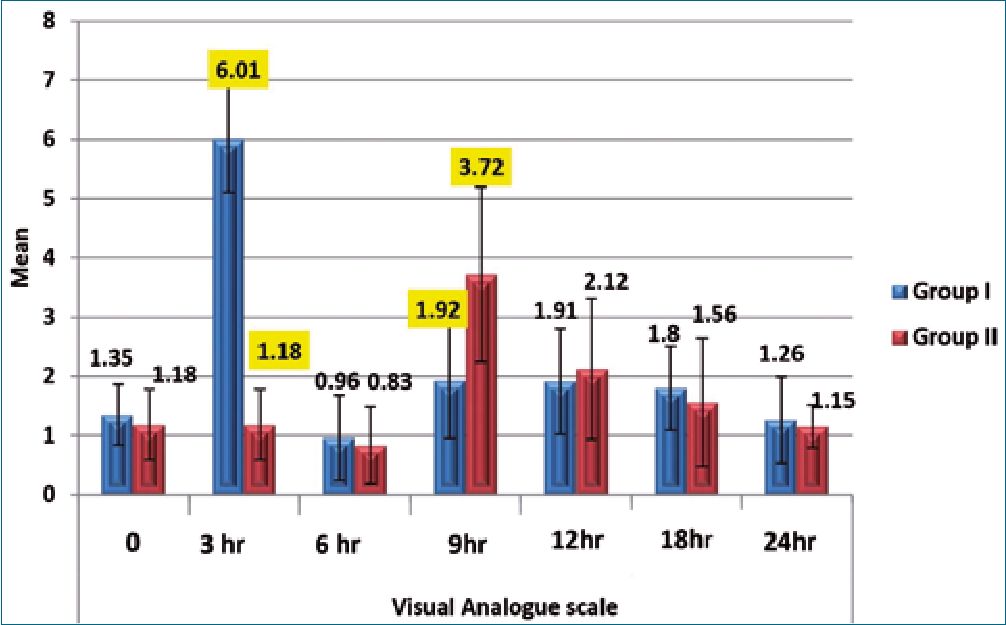
Figure 2. Visual Analogue scale among the studied groups.
In the current study, SC nalbuphine injection was associated with more sedation. Surprisingly enough, this feature was reported by most of the patients to be satisfying. They reported that they wished to be not vigilant during the early postoperative phase. Also, the postoperative sedation state did not represent a major disadvantage in our study population, it may warrant careful patient selection. Although there was more sedation after SC nalbuphine, however, we did not observe any single postoperative respiratory depression episode. This may be explained by the fact that respiratory depression is predominantly 3p receptor-mediated[24].
Similarly, Minai et al[25], reported significantly better intraoperative and postoperative analgesia with a longer time interval between reversal of neuromuscular paralysis and the patient’s ability to tell his/her full name in conjunction with fewer side effects for nalbuphine than morphine (16.2 and 11.3 min) respectively, they did not consider it a serious disadvantage in their clinical settings.
To the best of our knowledge, this is the first study to compare the analgesic efficacy of SC nalbuphine to other analgesic modalities also, the cost-effective management protocol for postoperative analgesia after open abdominal surgeries, which could be of great benefit in resource-limited areas. However, this study has some limitations. First, this is a single-center trial that assessed the analgesia profile in open abdominal surgeries. Second, this is a single blinded study in the operating room only, because the epidural group received an epidural catheter while the subcutaneous nalbuphine group did not receive. Third, exclusion of the patients with ASA physical status classes > II could limit the external validity and the generalizability of the current findings in this trial. Fourth, we did not assess the level of sensory block in epidural group patients after catheters insertion. Fifth, we did not use patient-controlled analgesia techniques due to limited resources. Alternatively, the titration method for postoperative analgesics supplement according to visual analogue scale used. Hence further research is warranted before generalizing the results.
Table 3. Sedation score, and satisfaction score among the studied groups
| Studied variables | Group I | Group II | Test of sig. | P value |
| At 0 min | Epidural analgesia (n = 65) | Subcutaneous nalbuphine (n = 65)
Sedation score |
||
| Mean ± SD
Range At 10 min |
2.56 ± 0.49
2 – 3 |
4.73 ± 0.44
4 – 5 |
t-test = 26.2 | 0.001** |
| Mean ± SD
Range At 20 min |
2.47 ± 0.50
2 – 3 |
4.09 ± 0.67
3 – 5 |
t-test = 15.4 | 0.001** |
| Mean ± SD
Range At 30 min |
2.36 ± 0.48
2 – 3 |
3.51 ± 0.70
2 – 5 |
t-test = 10.7 | 0.001** |
| Mean ± SD
Range Mild |
2.18 ± 0.39
2 – 3 11 (16.9) |
2.83 ± 0.74
2 – 4 Satisfaction score 0 (0.00) |
t-test = 6.21 | 0.001** |
| Good
Excellent |
54 (83.1)
0 (0.00) |
13 (20.0)
52 (80.0) |
X2 88.1 | < 0.001 |
Significance level at p-value < 0.05; **: High significance. x2: Chi-square test.
-
Conclusion
In patients scheduled for open abdominal surgeries, SC nalbuphine confers a favorable postoperative analgesic state in terms of first call for analgesics and opioid consumption with no obvious side effects. It may be an effective low-cost alternative to epidural analgesia in low-resource settings and in patients in whom epidural insertion is contraindicated or has already failed.
Conflicting Interest : None.
References
1. Pöpping DM, Elia N, Van Aken HK, Marret E, Schug SA, Kranke P, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014 Jun;259(6):1056–67. https://doi.org/10.1097/SLA.0000000000000237 PMID:24096762
2. Ariza F, Rodriguez-Mayoral H, Villarreal K. Epidural analgesia in abdominal major surgery: pros, cons, and unresolved issues beyond pain control. Rev Colomb Anestesiol. 2018;46(2):175–6. https://doi.org/10.1097/CJ9.0000000000000033.
3. Wagemans MF, Scholten WK, Hollmann MW, Kuipers AH. Epidural anesthesia is no longer the standard of care in abdominal surgery with ERAS. What are the alternatives? Minerva Anestesiol. 2020 Oct;86(10):1079–88. https://doi.org/10.23736/S0375-9393.20.14324-4 PMID:32420713
4. Zeng Z, Lu J, Shu C, Chen Y, Guo T, Wu QP, et al. A comparision of nalbuphine with morphine for analgesic effects and safety : meta-analysis of randomized controlled trials. Sci Rep. 2015 Jun;5(1):10927. https://doi.org/10.1038/srep10927 PMID:26039709
5. Życzkowska J, Wordliczek J. Subcutaneous and intravenous administration of analgesics in palliative medicine. Adv Palliat Med. 2009;8(4):153–60.
6. Sessler CN, Grap MJ, Ramsay MA. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12(Suppl 3 Suppl 3):S2. https://doi.org/10.1186/cc6148 PMID:18495053
7. Yassin HM, Abd Elmoneim AT, El Moutaz H. The Analgesic Efficiency of Ultrasound-Guided Rectus Sheath Analgesia Compared with Low Thoracic Epidural Analgesia After Elective Abdominal Surgery with a Midline Incision: A Prospective Randomized Controlled Trial. Anesth Pain Med. 2017 Jun;7(3):e14244. https://doi.org/10.5812/aapm.14244 PMID:28856110
8. Schmauss C, Doherty C, Yaksh TL. The analgetic effects of an intrathecally administered partial opiate agonist, nalbuphine hydrochloride. Eur J Pharmacol. 1982 Dec;86(1):1–7. https://doi.org/10.1016/0014-2999(82)90389-2 PMID:6897635
9. Tiwari AK, Tomar GS, Agrawal J. Intrathecal bupivacaine in comparison with a combination of nalbuphine and bupivacaine for subarachnoid block: a randomized prospective double-blind clinical study. Am J Ther. 2013;20(6):592–5. https://doi.org/10.1097/MJT.0b013e31822048db PMID:21904194
10. Gupta K, Rastogi B, Gupta PK, Singh I, Bansal M, Tyagi V. Intrathecal nalbuphine versus intrathecal fentanyl as an adjuvant to 0.5% hyperbaric bupivacaine for orthopedic surgery of lower limbs under subarachnoid block: A comparative evaluation. Indian J Pain. 2016;30(2):90–5. https://doi.org/10.4103/0970-5333.186463.
11. Rao ZA, Choudhri A, Naqvi S, Ehsan-Ul-Haq. Walking epidural with low dose bupivacaine plus tramadol on normal labour in primipara. J Coll Physicians Surg Pak. 2010 May;20(5):295–8. PMID:20642918
12. Kim TH, Kim JM, Lee HH, Chung SH, Hong YP. Effect of nalbuphine hydrochloride on the active phase during first stage of labour: a pilot study. J Obstet Gynaecol. 2011 Nov;31(8):724–7. https://doi.org/10.3109/01443615.2011.602139 PMID:22085063
13. Praveen PV, Konda VC, Lohit K. A prospective, randomized, double-blind, comparative study of intramuscular nalbuphine hydrochloride, butorphanol tartrate, and pentazocine lactate for postoperative pain relief following abdominal hysterectomy. Int J Basic Clin Pharmacol. 2016;5:2326–31.
14. Chandrakar N, Lalwani J, Sahare KK. Use of patient-controlled analgesia using I.V. Tramadol and I.V. Nalbuphine for postoperative pain management after major abdominal surgery – a comparative study. Int J Res Rev. 2016;3(5):43–53.
15. Ackerman AL, O’Connor PG, Doyle DL, Marranca SM, Haight CL, Day CE, et al. Association of an Opioid Standard of Practice Intervention With Intravenous Opioid Exposure in Hospitalized Patients. JAMA Intern Med. 2018 Jun;178(6):759–63. https://doi.org/10.1001/jamainternmed.2018.1044 PMID:29799964
16. Parsons HA, Shukkoor A, Quan H, Delgado-Guay MO, Palmer JL, Fainsinger R, et al. Intermittent subcutaneous opioids for the management of cancer pain. J Palliat Med. 2008 Dec;11(10):1319–24. https://doi.org/10.1089/jpm.2008.0155 PMID:19115891
17. Solanki RN, Gosai ND, Joshi GM, Patel BM, Modi HV, Jain R. A Comparative Study of Intravenous Nalbuphine HCl and Tramadol HCl for Postoperative Pain Relief Following Orthopaedic Surgeries. Asian Pac J Health Sci. 2015;2(1):155–60. https://doi.org/10.21276/apjhs.2015.2.1.26.
18. Kumar J, Sinha PK, Prasad BK. Comparative Study of Nalbuphine and Tramadol for Postoperative Pain Relief in Patients of Short Surgical Procedures under TIVA. Int J Contemp Med Res. 2017;4(4):977–99.
19. Hari Prasad K, Ram Kumar PA, Rajagokilam R. VinithraVaradarajan. A Comparative Study of Analgesic Potential of Nalbuphine Versus Fentanyl during General Anaesthesia. Int J Contemp Med Res. 2016;3(10):2815–7.
20. Sadafule NN, Karhade SS. Comparative Study of Efficacy of Preoperative Nalbuphine Hydrochloride and Pentazocine Lactate on Hemodynamic Response to Tracheal Intubation and Postoperative Analgesia. Anesth Essays Res. 2018;12(1):218–22. https://doi.org/10.4103/aer.AER_168_17 PMID:29628585
21. Borzellino G, Francis NK, Chapuis O, Krastinova E, Dyevre V, Genna M. Role of epidural analgesia within an ERAS program after laparoscopic colorectal surgery: a review and meta-analysis of randomized controlled studies. Surg Res Pract. 2016;2016:7543684. https://doi.org/10.1155/2016/7543684 PMID:27642630
22. Ladha KS, Patorno E, Liu J, Bateman BT. Impact of Perioperative Epidural Placement on Postdischarge Opioid Use in Patients Undergoing Abdominal Surgery. Anesthesiology. 2016 Feb;124(2):396–403. https://doi.org/10.1097/ALN.0000000000000952 PMID:26575145
23. Nimmo SM, Harrington LS. What is the role of epidural analgesia in abdominal surgery? Continuing Education in Anaesthesia. Critical Care & Pain J. 2014;14(5):224–9.
24. Dahan A, van der Schrier R, Smith T, Aarts L, van Velzen M, Niesters M. Averting Opioid-induced Respiratory Depression without Affecting Analgesia. Anesthesiology. 2018 May;128(5):1027–37. https://doi.org/10.1097/ALN.0000000000002184 PMID:29553984
25. Minai FN, Khan FA. A comparison of morphine and nalbuphine for intraoperative and postoperative analgesia. J Pak Med Assoc. 2003 Sep;53(9):391–6. PMID:14620312

 ORCID
ORCID

 Creative Commons Attribution
Creative Commons Attribution