Alejandro Placenti MD.1, Stella Verlangieri MD.1, Daniela Porticella MD.1, Santiago Tau Anzoategui MD.1,*
Recibido: 07-07-2023
Aceptado: 22-09-2023
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 8 pp. 836-839|https://doi.org/10.25237/revchilanestv52n8-22
PDF|ePub|RIS
Evaluación ecocardiográfica de colapso hemodinámico durante una neurocirugía
Abstract
Hemodynamic collapse it is not rare in the operating room. A 78-year-old man undergoes a resection for a right temporal meningioma. Shortly after induction, the patient goes into shock and pulmonary embolism (PE) is suspected. Transthoracic echocardiography shows hyperechoic material in the right pulmonary artery that is compatible with a thrombus. The PE diagnosis is confirmed and the surgery is suspended. Once again, point-of-care ultrasound (POCUS) shows us that daily application is crucial in our anesthesiological practice. We must know the different thromboprophylaxis approaches that we can apply in perioperative patients with high risk of thrombovenous disease.
Resumen
El colapso hemodinámico no es infrecuente en quirófano. Un hombre de 78 años se somete a una resección de un meningioma temporal derecho. Poco después de la inducción, el paciente entra en estado de shock y se sospecha una embolia pulmonar (EP). La ecocardiografía transtorácica muestra un material hiperecoico en la arteria pulmonar derecha compatible con un trombo. Se confirma el diagnóstico de EP y se decide suspender la cirugía. Una vez más, el “Ultrasonido Point of Care” (POCUS) nos demuestra que su aplicación diaria es crucial en nuestra práctica anestesiológica. En pacientes con alto riesgo de enfermedad tromboembólica es importante conocer los diferentes arsenales profilácticos y terapéuticos para evitar que se desencadene un desenlace no deseado como el que sucedió en este caso.
-
Introduction
Pulmonary embolism (PE) is a frequent cause of morbidity and mortality, and its incidence decreases significantly if adequate prevention measures are carried out. In this neurosurgical anesthesia case, we can observe how important is to develop and practice adequate echocardiographic skills to diagnose a pulmonary embolism. We can find different echocardiographic signs, most of them are the consequences of pressure overload in the right ventricle. In this specific case we observed the thrombus directly but this is not a common finding so we must learn other echocardiographic signs to make a diagnosis of pulmonary embolism.
-
Case report
A 78-year-old male patient was scheduled for a resection of a right temporal meningioma. His medical history presented: atrial fibrillation, arterial hypertension, diabetes, prostate cancer, and depressive syndrome. About 15 minutes after induction, the patient developed hypotension of 89/51 mm Hg, Spo2 of 85%, and tachycardia of 105 bpm without changes in capnography or airway pressures. The inspired fraction of oxygen is raised to 100% and the noradrenaline dose is increased gradually to a maximum of 0.2 mcg • kg-1 • min-1.
A transthoracic echocardiographic examination is then performed, starting in the right parasternal long-axis view (PLAX) where right ventricle (RV) dilatation is observed (Figure 1). A parasternal short-axis view (PSAX) at the level of the pulmonary artery bifurcation shows a mobile hyperechoic image compatible with a thrombus that was in the right pulmonary artery (Figure2). In PSAX at papillary muscles level, septal flattening is observed with the “D sign” pattern. A tricuspid annulus plane systolic excursion (TAPSE) was measured with a value of 14mm. The inferior vena cava averaged 2.2 cm without signs of collapse in the subcostal view estimating the right atrial pressure of 20 mm Hg. The systolic pressure in the pulmonary artery (PSAP) was 66 mm Hg (Figure 3). Another image compatible with a thrombus was obtained in a RV outflow view.
The diagnosis of massive PE was confirmed as a result of the images obtained. It was decided to suspend the surgical procedure and the patient was transferred to the intensive care unit. The electrocardiogram showed S1Q3T3 pattern. Consequently, the laboratory evidenced the following: Troponin I 41 ng / L, CK 26 IU / L, Nt pro-BNP 1403 pg / ml. An infusion of low molecular weight heparin guided by kinetic partial thromboplastin time was decided, since the patient had an absolute contraindication to thrombolytics due to the primary tumor in the central nervous system. Two hours after the event, no vasoactive was required to maintain blood pressure in normal ranges. He was extubated without complications within 24 hours. Rivaroxaban was indicated as long-term anticoagulant therapy and hospital discharge was performed after 5 days.
-
Discussion
In the context of programmed neurosurgery, a thromboembolic event with an acute corpulmonale (sudden and acute increase in the afterload of the RV) may put the patient’s life at risk.
This patient had high risk of bleeding and high risk of thrombosis so an multi-disciplinary approach is needed previous to the surgery. A patient that is programmed for a craniectomy it is included in high-risk bleeding patients. We must take in account that he had the risk of arterial thromboembolism from his previous atrial fibrillation and in the other hand he had high risk of venous thromboembolism with a Caprini score of 8 points that correspond to high-risk patients of having an event mentioned above. A patient with these characteristics needs perioperative mechanical thromboprophylaxis but probably not pharmacological prophylaxis because of the high-risk bleeding. Transthoracic echocardiography may assist us to diagnose PE. However, in the absence of signs of the latter, we might be inclined to diagnose different shock conditions such as pericardial tamponade, acute valve dysfunction, left ventricle (LV) wall motion abnormalities, aortic dissection, or hypovolemia.
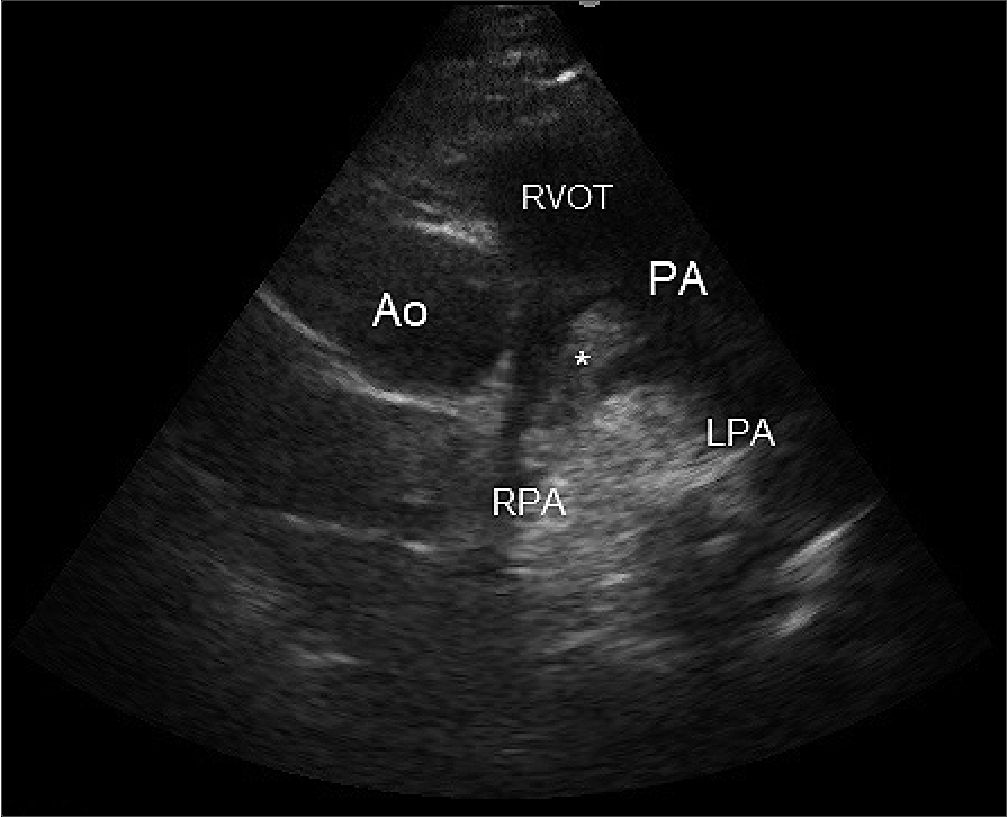
Figure 2. In an apical four chamber view (A4C) we can measure with continuous doppler (CW) the maximum velocity of tricuspid regurgitation (TR). Footnotes A: corresponds to peak velocity expressed in cm – seg-1 Gr p: corresponds to the pressure gradient between right atrial and right ventricle.
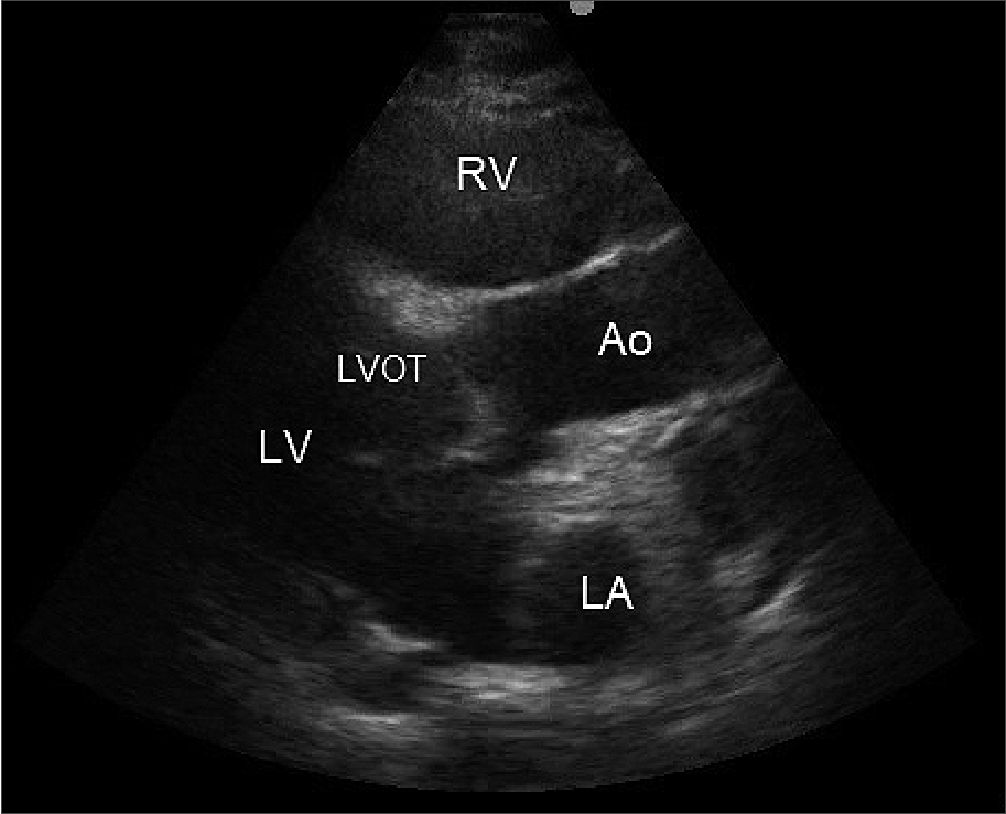
Figure 1. In a parasternal long-axis view (PLAX) we can see dilatation of the right ventricle (RV) that could be explained by different causes.Foot notes RV: right ventricle; Ao: Aorta; RA: right atrium; LV: Left ventricle; LVOT: Left ventricle outflow tract.
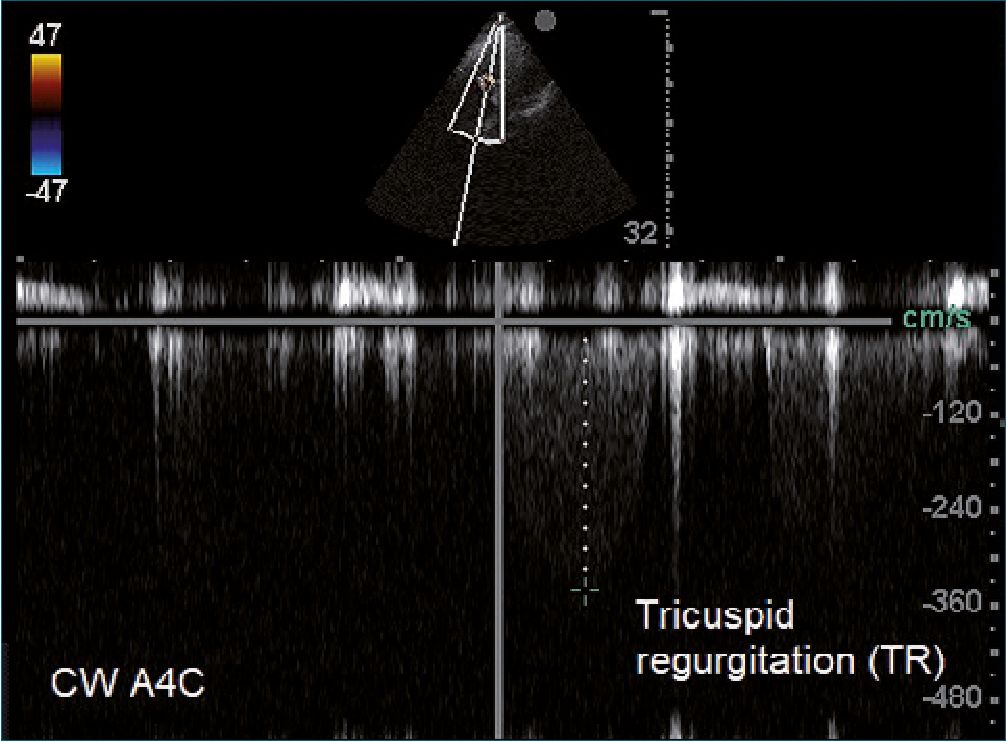
Figure 3. In a parasternal short-axis at pulmonary artery bifurcation (PSAX-PAB) we can observe directly the thrombus (asterisk) lodged in the right pulmonary artery. Footnotes Ao: Aorta; RVOT: right ventricular outflow tract; PA: pulmonary artery; RPA: right pulmonary artery; LPA: left pulmonary artery.
The case shows us the importance of intraoperative echocardiography performed by an anesthesiologist, for the diagnosis of high-risk (massive) PE. Indeed, we sometimes recognize a shock condition without knowing what type it is, which leads us to take empirical measures such as increasing the dose of vasoactive agents, the infusion of fluids and the inspired fraction of oxygen, etc. In the case described, augmenting fluids infusion would have been deleterious, worsening the cardiovascular collapse. Echocardiography plays a fundamental role in these scenarios as an “eyeball” and it is not strictly necessary to take specific measurements on the different cavities, thicknesses, velocities, and the like. Transthoracic echocardiography in critically ill patients is merely different from those performed in an ambulatory setting because mechanical ventilation, drains, sterile areas, position, among others, makes the examination difficult. As many tools we use in monitoring and diagnosis, it is important to place them in context and integrate this information with other monitors.
Measuring RV function in a critical patient with mechanical ventilation is actually a challenge.Beyond identification of thrombus in the right side of the heart, echocardiography is useful in assessing the effect of PE on cardiac function, particularly RV function.
The RV is made up of thin muscular layers arranged longitudinally to the axis of the heart that make it very adaptable to changes in volume, except to acute changes in pressure[1]. Its low capacity to adapt to the increase in pulmonary vascular resistance generates the dilatation of the RV. This results in less contractility, a septal shift that decreases the LV diastolic diameter (ventricular interdependence) and tricuspid regurgitation in some cases. Therefore, LV generates a lower systolic volume that ends up decreasing the coronary perfusion pressure. Right ventricular ischemia can lead to an even lower systolic volume and enter the so-called right ventricular death spiral[2]. The decrease in right systolic volume can lead to right ventricular ischemia even in the absence of coronary disease, which would worsen the scenario[1].
RV size can be estimated from A4C, PSAX, and subcostal views. Under normal conditions, from the AC4 view, RV is triangular and smaller than the LV (the ratio between the area of the RV and the normal LV is < 0.6)[3].
In PSAX view, the anteroposterior diameter of the RV should measure between 20 to 30 mm. It is important to place the probe correctly because if it is placed too high or otherwise to the right, we may overestimate the size of the right cavities. In addition to size, the thickness of walls should be evaluated[4]. A thickness greater than 5 mm of the RV free wall measured at the end of the diastole is consistent with RV hypertrophy[2]. The free wall of the RV is made up of longitudinal and oblique fibers that, when shortened longitudinally like a piston, constitute approximately 80% of the right myocardial function. TAPSE from an A4C view estimates the lateral wall displacement from the base to the ventricular apex[5]. TAPSE values > 17 mm indicate good ventricular function. In fact, a value below is an indicator of RV systolic failure[2].The Mc connell’s sign described as hyperkinesia in the apex and hypokinesia or akinesia of the free wall is less frequent to observe but, when found, it has a high positive predictive value for PE[6].Measurement of PSAP is possible in more than 80% of transthoracic echocardiography examination[2]. The pressure gradient between RV and right atrial is calculated through the maximum velocity of tricuspid regurgitation using Bernoulli simplified equation. When the estimated value of right atrial pressure (measuring the diameter of the inferior vena cava) is added to maximum velocity of tricuspid regurgitation, the value of PSAP is obtained. The low capacity of RV to eject compared to the high afterload explains why in acute scenarios the PSAP is usually < 60 mm Hg. The PSAP value with the acceleration time in the pulmonary valve < 60 milliseconds make up the “60/60 sign” that, together with the mid systolic notch, has a high positive predictive value for detecting acute PE when the clinical suspicion is high[7]. In a study by Katarzyna Kurnicka, up to 68.8% of high-risk PEs observed flattening of the interventricular septum in a PSAX papillary muscles level[8]. It is useful to measure the peak systolic (S ‘) velocity of tricuspid annulus, indeed. A peak velocity of tricuspid annulus > 10.5 cm – seg-1 identified individuals with both a normal RV function and without significant pulmonary hypertension [9].A mobile thrombus in the right chambers confirms the diagnosis of PE and is associated with increased mortality, especially if the patient has RV dysfunction[10].Common findings coexisting with acute PE mentioned above are summarized in Table 1 (Table 1).
| Table 1. Echocardiographic features in pulmonary embolism |
| RV* dilatation |
| RV* dysfunction (TAPSE+ / peak systolic velocity of tricuspid annulus / wall motion abnormalities) |
| Interventricular septal flattening or “D sign” |
| Tricuspid regurgitation |
| Attenuation of normal collapse of inferior vena cava |
| Pulmonary hypertension (60/60 sign) |
| Direct visualization of thrombus |
| *RV: right ventricle; +: TAPSE: tricuspid annulus plane systolic excursion. |
These patients present high risk of thrombosis and bleeding so a multi-disciplinary perioperative approach is needed. Without the use of ultrasound, this patient’s therapeutic measures would probably have been taken without an accurate diagnosis and without support of scientific evidence. We must use ultrasound in our anesthesiologic practice daily.
Acknowledgments: The authors sincerely thank the public translator Belen Carminatti for her help in relation to the use of the English language.
Los autores agradecen sinceramente a la traductora publica Belen Carminatti por su ayuda con relación a la utilización de la lengua inglesa.
La presente investigación no ha recibido ayudas específicas provenientes de agencias del sector público, sector comercial o entidades sin ánimo de lucro.
-
References
1. Ventetuolo CE, Klinger JR. Management of acute right ventricular failure in the intensive care unit. Ann Am Thorac Soc. 2014 Jun;11(5):811–22. https://doi.org/10.1513/AnnalsATS.201312-446FR PMID:24828526
2. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al.; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020 Jan;41(4):543–603. https://doi.org/10.1093/eurheartj/ehz405 PMID:31504429
3. Vieillard-Baron A. Assessment of right ventricular function. Curr Opin Crit Care. 2009 Jun;15(3):254–60. https://doi.org/10.1097/MCC.0b013e32832b70c9 PMID:19451815
4. Hockstein MA, Haycock K, Wiepking M, Lentz S, Dugar S, Siuba M. Transthoracic Right Heart Echocardiography for the Intensivist. J Intensive Care Med. 2021 Sep;36(9):1098–109. https://doi.org/10.1177/08850666211003475 PMID:33853435
5. Brown SB, Raina A, Katz D, Szerlip M, Wiegers SE, Forfia PR. Longitudinal shortening accounts for the majority of right ventricular contraction and improves after pulmonary vasodilator therapy in normal subjects and patients with pulmonary arterial hypertension. Chest. 2011 Jul;140(1):27–33. https://doi.org/10.1378/chest.10-1136 PMID:21106653
6. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996 Aug;78(4):469–73. https://doi.org/10.1016/S0002-9149(96)00339-6 PMID:8752195
7. Kurzyna M, Torbicki A, Pruszczyk P, Burakowska B, Fijałkowska A, Kober J, et al. Disturbed right ventricular ejection pattern as a new Doppler echocardiographic sign of acute pulmonary embolism. Am J Cardiol. 2002 Sep;90(5):507–11. https://doi.org/10.1016/S0002-9149(02)02523-7 PMID:12208411
8. Kurnicka K, Lichodziejewska B, Goliszek S, et al. Embolgraphic Pattern Analysis of Acutechomonic Analysis of Pulmonary Embolism P. 511 Consecutive Patients. J Am Soc Echocardiogr. 2016 Sep;29(9):907–13. https://doi.org/10.1016/j.echo.2016.05.016 PMID:27427291
9. Saxena N, Rajagopalan N, Edelman K, López-Candales A. Tricuspid annular systolic velocity: a useful measurement in determining right ventricular systolic function regardless of pulmonary artery pressures. Echocardiography. 2006 Oct;23(9):750–5. https://doi.org/10.1111/j.1540-8175.2006.00305.x PMID:16999693
10. Koć M, Kostrubiec M, Elikowski W, Meneveau N, Lankeit M, Grifoni S, et al.; RiHTER Investigators. Outcome of patients with right heart thrombi: the Right Heart Thrombi European Registry. Eur Respir J. 2016 Mar;47(3):869–75. https://doi.org/10.1183/13993003.00819-2015 PMID:26797032

 ORCID
ORCID

 Creative Commons Attribution
Creative Commons Attribution