Tarek Samir Shabana MD.1,*, Safaa Ishak Ghaly MD.1, DiaaElDein Mahmoud Ibrahim MD.2
Recibido: 10-03-2023
Aceptado: 10-09-2023
©2024 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 53 Núm. 1 pp. 52-59|https://doi.org/10.25237/revchilanestv53n1-10
PDF|ePub|RIS
Abstract
Duration of analgesia with incisional infiltration using local anesthetics is short. Ketamine and dexamethasone are known for their role as adjuvants to local anesthetics. The present study compared the effects of ketamine and dexamethasone on the duration of analgesia when combined with bupivacaine for incisional infiltration in pediatric abdominal operations. A total of 150 children (1-8 years) undergoing open abdominal operations were randomized equally into three groups to receive incisional infiltration with Bupivacaine 0.25% 1 ml/kg (GpB), ketamine 1 mg/kg + bupivacaine (GpK), and dexamethasone 0.2 mg/kg + bupivacaine (GpD). Groups were compared for time for first analgesic dose (primary outcome), number of children requiring rescue analgesia; number of rescue analgesia doses; and FLACC scores (in the first 24 h postoperatively). Time for first analgesic dose was significantly longer with ketamine (7.7 ± 2.41 h) and dexamethasone (7.46 ± 2.77 h) compared to bupivacaine (3.44 ± 1.34 h) (P < 0.001). FLACC scores were significantly lower in the dexamethasone and ketamine groups compared to bupivacaine at 4 h, 6 h, 8 h, 10 h and 12 h postoperatively (P < 0.001). Forty-eight children in the bupivacaine group required rescue analgesia which was significantly higher than the ketamine and dexamethasone groups (36 and 29 children respectively, P < 0.001. Combining either ketamine or dexamethasone with bupivacaine for incisional infiltration prolonged the duration of analgesia after pediatric abdominal operations.
Resumen
La duración de la analgesia con infiltración de la incisión usando anestésicos locales es corta. La ketamina y la dexametasona son conocidas por su papel como adyuvantes de los anestésicos locales. El presente estudio comparó los efectos de la ketamina y la dexametasona sobre la duración de la analgesia cuando se combinaron con bupivacaína para la infiltración incisional en operaciones abdominales pediátricas. Un total de 150 niños (1-8 años) sometidos a operaciones abdominales abiertas fueron aleatorizados en tres grupos para recibir infiltración con bupivacaína 0,25% 1 ml/kg (GpB), ketamina 1 mg/kg + bupivacaína (GpK) y dexametasona 0,2 mg/kg + bupivacaína (GpD). Los grupos se compararon por tiempo para la primera dosis analgésica de rescate (resultado primario), número de niños que requirieron analgesia de rescate; número de dosis de analgesia de rescate; y puntuaciones FLACC (en las primeras 24 h del posoperatorio). El tiempo para la primera dosis analgésica fue significativamente mayor con ketamina (7,7 ± 2,41 h) y dexametasona (7,46 ± 2,77 h) en comparación con bupivacaína (3,44 ± 1,34 h) (P < 0,001). Las puntuaciones FLACC fueron significativamente más bajas en los grupos de dexametasona y ketamina en comparación con bupivacaína a las 4 h, 6 h, 8 h, 10 h y 12 h después de la operación (P < 0,001). Cuarenta y ocho niños en el grupo de bupivacaína requirieron analgesia de rescate que fue significativamente mayor que los grupos de ketamina y dexametasona (36 y 29 niños respectivamente, P < 0,001. La combinación de ketamina o dexametasona con bupivacaína para la infiltración incisional prolongó la duración de la analgesia después de operaciones abdominales pediátricas.
-
Introduction
Pain after open abdominal surgery in children is significant and multimodal approach is necessary for proper pain management. Owing to concerns about side effects of opioid administration and difficulties in pain assessment, children are still subject to suboptimal pain management at many centers[1].
Incisional infiltration with local anesthetics is an affordable and simple method of providing postoperative pain relief that has endured competition with other modalities for postoperative pain management[2].
When injected into the surgical wound, local anesthetics inactivate sodium channels in peripheral nerve endings, thus preventing impulse propagation along these nerves[3].
The main limitation of this technique is the short duration of analgesia. For this reason, many adjuvants (as a2 agonists, opioids and magnesium) have been used in combination with local anesthetics to increase the magnitude and duration of analgesia[4].
Ketamine is a popular intravenous anesthetic that produces dissociative anesthesia by blocking N-Methyl D-Aspartate (NMDA) receptors. Ketamine is also known for its potent analgesic effects and its role as an adjuvant to local anesthetics has been demonstrated in previous studies[5].
Another drug possessing analgesic properties is dexamethasone which is well known for its potent anti-inflammatory effects. Many studies have revealed the prolongation in the duration of analgesia when dexamethasone was combined with local anesthetics in neuraxial anesthesia, peripheral nerve blocks and local infiltration in both children and adults[6].
Thus far, no previous study has compared the effects of ketamine and dexamethasone as adjuvants to local anesthesia for incisional infiltration.
The aim of this study was to compare the effects of both ketamine and dexamethasone on the duration of analgesia when combined with bupivacaine for incisional infiltration in pediatric abdominal operations.
-
Patients and Methods
After gaining institutional ethical clearance (FMASU R 122a/2020) from Faculty of Medicine, Ain Shams University and informed consent from the child’s parent or guardian, this prospective, randomized study was carried out in Ain Shams University Pediatric Surgery department on 150 children aged 1-8 years undergoing open abdominal operations. The study was registered in ClinicalTrials.gov NCT05190952. This clinical trial was in accordance with the standards of the Helsinki Declaration (1975, revised in 2013).
Children were randomized using computer generated random numbers into three groups of 50 patients each. Group B: Bupivacaine 0.25% 1 ml/kg was injected subcutaneously before wound closure. Group K: in addition to bupivacaine, ketamine 1 mg/kg (diluted in 10 ml normal saline) was injected subcutaneously before wound closure. Bupivacaine and ketamine were administered using two separate syringes. Group D: in addition to bupivacaine, dexamethasone 0.2 mg/kg (diluted in 10 ml normal saline) was injected subcutaneously before wound closure. Bupivacaine and dexamethasone were administered using two separate syringes. CONSORT flow diagram is presented in Figure 1.
The primary outcome was the time needed for the first analgesic dose. Secondary outcomes included need for rescue analgesia (number of children requiring rescue analgesia and number of rescue analgesia doses in the first 24 h postoperatively), FLACC[7] pain scores (in the PACU, then 2 hourly for 12, and then 4 hourly for the next 12 hours), heart rate and mean blood pressure (at baseline [before infiltration], in the PACU, and then 2 hourly for 24 h ), incidence of wound complications (over a period of 7 days) and incidence of postoperative nausea and vomiting (PONV).
For all groups, drugs were withdrawn into sterile syringes by the attending anesthesiologist and injected by the surgeon who was blinded to the allocation. Pain assessment in the PACU was done by an independent anesthesiologist and by a bedside nurse thereafter. Both were blinded to the study allocation.
-
First analgesic dose
If the FLACC scale was more than 3 either during intervals of FLACC recording or in between, intravenous paracetamol was provided at a dose of 15 mg/kg (10 mg/kg for children less than 10 kg) then fixed around the clock every 6 h.
-
Rescue analgesia
If pain persisted or recurred, rectal diclofenac 1mg/kg or ibuprophen 10 mg/kg (in children in whom oral intake has been allowed) were administered and repeated in 8 h if needed. In children with FLACC scores more than 6 (denoting severe pain), 0.5 pg/kg fentanyl IV was given in addition to NSAIDs.
Exclusion criteria included history of diabetes, cardiac or neurological disease, risk of wound complications (as infected wound, history of wound dehiscence, hypoalbuminemia), multiple incision sites, and cases scheduled as day-cases.
-
Anesthetic technique
In all children, anesthesia was induced by sevoflurane (8%) followed by insertion of an IV line or propofol 2mg/kg IV (premixed with lidocaine 0.1%) in children arriving to the OR with an IV line. Fentanyl 2 pg /kg and atracurium 0.5 mg/kg were then administered followed by endotracheal intubation and mechanical ventilation. Anesthesia was maintained with sevo- flurane in oxygen.MAC levels were adjusted according to age and autonomic responses (MAC-BAR). Additional boluses of 0.5-1 pg/kg fentanyl were administered in the first hour of surgery in children with heart rates remaining above 20% of baseline after exclusion of other possible causes of tachycardia. No other analgesics were given intraoperative. All children were monitored using ECG, non-invasive blood pressure cuff, pulse oximeter and temperature probe. Ringer acetate was used as the replacement fluid. At the end of surgery, the trachea was extubated after reversal of the muscle relaxant with neostigmine 0.05 mg/kg + atropine 0.02 mg/kg and the child transferred to the PACU then to the surgical ward or the intensive care unit.
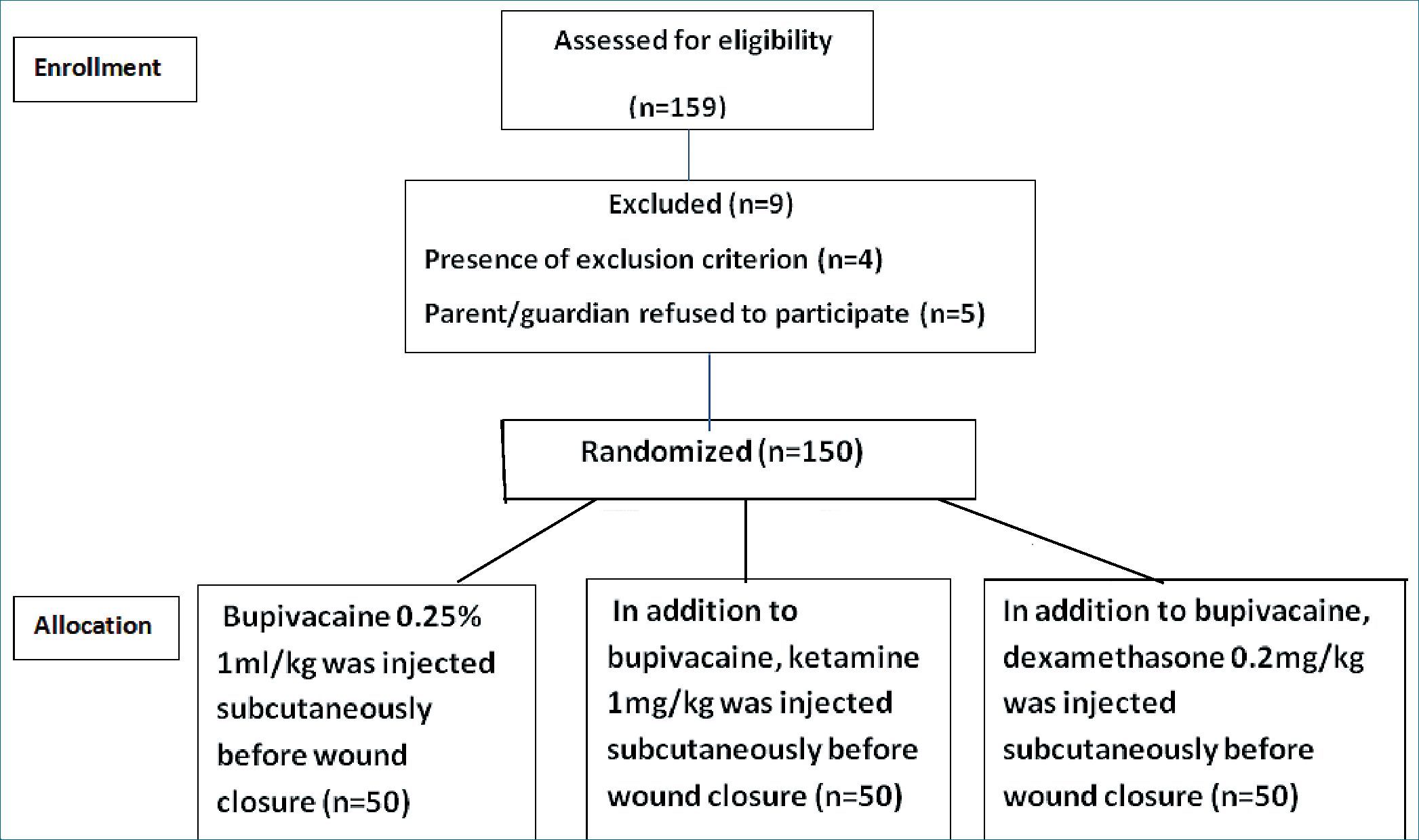
Figure 1. CONSORT Flow Diagram.
Sample size calculation
The sample size was calculated using PASS program version 15, setting alpha error at 5% and power at 95%. Results from a previous study Mohamed et al., 2018[8] showed that among ketamine group the time to receive analgesia was 7.6 ± 4.1 h compared to 4.2 ± 2.1 h in bupivacaine group and according to Subhedar et al., 2016 [9] the mean time for dexamethasone group was 4.3 ± 0.8 h. Based on this data a minimal sample of 38 cases per group was needed. However, the sample size was set at 50 cases per group do compensate for any possible dropouts (Sampling Method: simple random sampling).
Statistical methods
SPSS (V.210.0, IBM Corp., USA, 2015) was used for data analysis. Categorical data were analyzed using the chi-square test. Numerical data were analyzed using Analysis of variance (ANOVA) and Student’s t-test as post-hoc test. Quantitative parametric data were expressed as mean (standard deviation, SD). Non-normally distributed data was presented as median (Interquartile range: first quartile-third quartile) and compared non-parametrically using Mann-Whitney U-test. Significance was set at P < 0.05.
-
Results
There were no significant differences between the three groups with regard to age, sex, weight, type of operation and duration of surgery, P > 0.05 (Tables 1 and 2).
Time for first analgesic dose was significantly longer with ketamine (7.7 ± 2.418 h) and dexamethasone (7.46 ± 2.77 h) compared to bupivacaine (3.44 ± 1.34 h) (P < 0.001). Fortyeight children in the bupivacaine group required rescue analgesia in the first 24 h postoperatively which was significantly less than the ketamine and dexamethasone groups (36 and 29 children respectively, P < 0.001). Ketamine and dexamethasone groups are comparable as regards for time for first analgesic dose (P = 0.64) and number of children requiring rescue analgesia (P = 0.09) (Table 3).
Analysis of number of children requiring rescue analgesia showed a significantly smaller number of children requiring 2 doses of rescue analgesia in ketamine (3 children) and dexamethasone (1 child) groups compared to bupivacaine group (19 children), P < 0.001. Two children in the bupivacaine group did not need rescue analgesia compared to 14 children in the ketamine group (P = 0.001) and 21 children in the dexamethasone group (P < 0.001).
FLACC scores were significantly lower in groups D and K compared to bupivacaine group at 4 h, 6 h, 8 h, 10 h and 12 h postoperatively, (P < 0.001) but comparable between groups K and D. In PACU and at 2 h, 16 h, 20 h and 24 h postoperatively, FLACC scores were comparable between groups B, K and D (Figure 2).
Groups B, K and D were comparable with regard to heart rate and mean blood pressure at baseline, in the PACU and at every 2 h for 24 h, P > 0.05 (Figures 3 and 4). There were no significant differences between groups with regard to the incidence of PONV in groups B (6%), K (8%), and D (6%) (P > 0.05) and no detected wound complications for all cases during the 7-day follow up.
1. Patient Demographics and duration of surgery
| Gp. B (n = 50) | Gp.K (n = 50) | GP.D (n = 50) | P-value | |
| Age (months) | 48.84 (25.63) | 47.68 (24.8) | 50.88 (25.2) | 0.81 |
| Sex(M:F) | 29:21 | 26:24 | 24:26 | 0.6 |
| Weight (kg) | 15.24 (4.59) | 16.98 (10.96) | 15.56 (4.68) | 0.45 |
| Duration of surgery (h) | 2.62(0.67) | 2.53(0.65) | 2.64(0.72) | 0.69 |
B: = Bupivacaine; K: = Ketamine; D: = Dexamethasone; Data presented as mean (standard deviation) or number (%).
Table 2. Type of abdominal surgery
| Gp.B(n = 50) | Gp.K (n = 50) | GP.D(n = 50) | |
| Type of surgery [N(%)] | |||
| Laparotomy | 10 (20%) | 9 (18%) | 11 (22%) |
| Nephrectomy | 3 (6%) | 4 (8%) | 3 (6%) |
| Pyeloplasty | 8 (16%) | 9 (18%) | 10 (20%) |
| Nissen fundoplication | 4 (8%) | 3 (6%) | 3 (6%) |
| Splenectomy | 2 (4%) | 3 (6%) | 3 (6%) |
| Hepatectomy | 1 (2%) | 1 (2%) | 0 (0%) |
| Closure of colostomy /ileostomy | 10 (20%) | 9 (18%) | 11 (22%) |
| Bladder augmentation | 3 (6%) | 2 (4%) | 2 (4%) |
| Appendectomy | 9 (18%) | 10 (20%) | 7 (14%) |
| B: = Bupivacaine; K: = Ketamine; D: | =Dexamethasone; Data presented as | number and percentage of patients. |
| Table 3. Time for first analgesic dose and need for rescue analgesia | ||||||
| Gp. B (n = 50) | Gp.K (n = 50) | GP.D (n = 50) | Gp B vs K | P- valueGp.B vs D | Gp.K vs D | |
| Time for first analgesic dose (h ) | 3.44 (1.34) | 7.7 (2.41) | 7.46 (2.77) | 0.00* | 0.00* | 0.64 |
| Number of children requiring rescue | 48 (96%) | 36 (72%) | 29 (58%) | 0.00* | 0.00* | 0.09 |
| analgesia [N (%)]Number of doses of rescue analgesia 0 | 2 | 14 | 21 | 0.001* | 0.00* | 0.14 |
| 1 | 24 | 32 | 27 | 0.1 | 0.06 | 0.72 |
| 2 | 19 | 3 | 1 | 0.00* | 0.00* | 0.3 |
| 3 | 5 | 1 | 1 | 0.09 | 0.09 | 1 |
*= P < 0.05; B; = Bupivacaine; K: = Ketamine; D: = Dexamethasone; Data presented as mean (standard deviation) or number (%).
-
Discussion
In the current study, administration of either ketamine or dexamethasone as adjuvants to bupivacaine for incisional infiltration in pediatric abdominal operations resulted in prolonged duration of postoperative analgesia evident by delayed time for first analgesic dose, decreased postoperative FLACC scores and a smaller number of children requiring rescue analgesia compared to bupivacaine alone.
To the best of our knowledge, no previous study has compared the effects of ketamine and dexamethasone as adjuvants to local anesthesia for perioperative wound infiltration. However, the beneficial effects on the quality of pain control provided by dexamethasone or ketamine when combined with local anesthetics have been supported by previous studies in both the
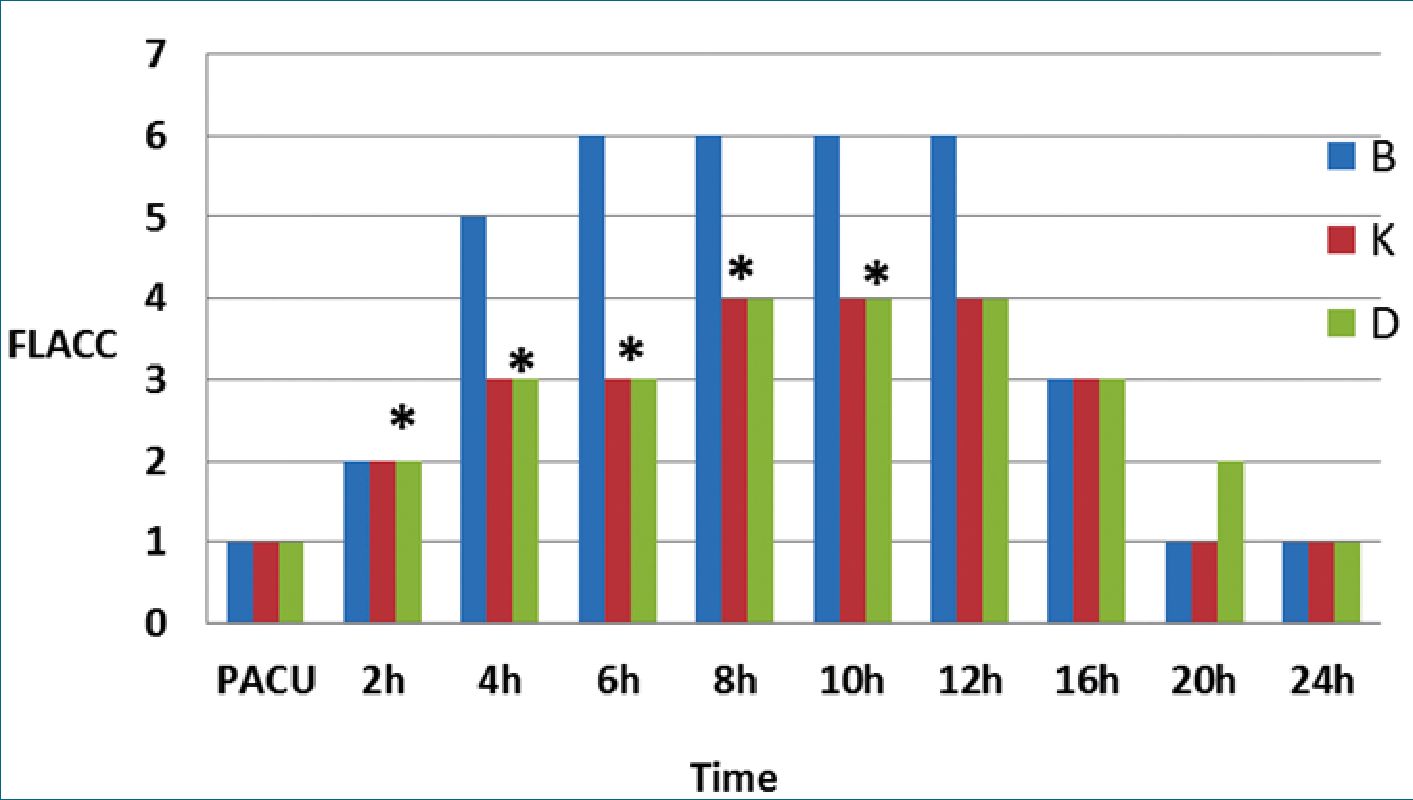
Figure 2. Postoperative median FLACC scores *= P < 0.05 Groups K vs B and Groups D vs B PACU = Post Anesthesia Care Unit; B: = Bupi- vacaine; K: = Ketamine; D: = Dexamethasone; FLACC = Face, Legs, Activity, Crying, Consolability.
Figure 3. Heart rate among the study groups. Error bars denote standard deviation. B: = Bupivacaine; K: = Ketamine; D: = Dexamethasone.
Figure 4. Mean blood pressure among the study groups. Error bars denote standard deviation. B: = Bupivacaine; K: = Ketamine; D: = Dexamethasone.
pediatric and adult populations.
The analgesic effects of dexamethasone can simply be attributed to the decreased production of pain mediators at nerve endings. Moreover, following peri-neural administration, dexamethasone acts on glucocorticoid receptors increasing the expression of inhibitory potassium channels resulting in membrane stabilization of nociceptive C fibers. The prolongation of the action of local anesthetics may be also due local vasoconstriction produced by local steroid injection. Furthermore, bupi- vacaine possesses pro-inflammatory effects that may be counteracted by the anti-inflammatory effects of dexamethasone. [10]-[11].
The results of the study by Subhedar et al[9] showed a delayed time for rescue analgesia when dexamethasone was used for wound infiltration in combination with lidocaine in day case surgery. Similarly, adding dexamethasone to ropivacaine resulted in reduced pain scores when used for scalp infiltration in craniotomy operations[12].
Evaristo-Mendez[13]. combined dexamethasone with ropi- vacaine for incisional infiltration during laparoscopic cholecystectomy resulting in decrease in the intensity of pain at 12 and 24 h postoperatively compared to ropivacaine alone. Moreover, combining dexamethasone with lidocaine for local infiltration before episiotomy resulted in significant pain relief[14].
Interestingly, incorporation of dexamethasone into extended-release bupivacaine microcapsules administered subcutaneously significantly increased the duration of local analgesia in human volunteers[15].
In contrast, the study carried by Rao et al., in 45 patients undergoing temporomandibular joint ankyloses surgery showed that 24 h pain control provided by ropivacaine wound infiltration was comparable to ropivacaine mixed with dexamethasone 0.1 mg/kg[16]. However, the sample size in the latter study was relatively small and the dose of dexamethasone was less than that used in the present study.
The results of the studies investigating the analgesic effects of peritonsillar dexamethasone infiltration in tonsillectomy surgeries are conflicting. Addition of dexamethasone to levobupi- vacaine for preoperative peritonsillar infiltration in children by Basuni et al.[17] improved postoperative analgesia compared to levobupivacaine infiltration + intravenous dexamethasone. On the other hand, in the study carried by Montazeri et al.[18], pre-incisional infiltration of tonsils did not reduce post-tonsillectomy pain in children compared to normal saline, perhaps because it was used as a sole analgesic agent not as an adjuvant.
There is no consensus about the optimal dose of dexamethasone for wound infiltration. Doses of 0.1 mg/kg in peripheral nerve blocks[19] and up to 0.2 mg/kg in caudal blocks[20] have been used successfully and safely. Although doses up to 0.5 mg/kg have been used for peritonsillar infiltration in chil- dren[18], we find this dose relatively high.
Ketamine is a dissociative anesthetic that antagonizes NMDA receptors. Through this mechanism of action, it also reduces central hyper-sensitization. Additionally, at small doses, ketamine interacts with opioid receptors. At therapeutic doses, ketamine also has local anesthetic properties through its ability to inhibit neuronal sodium channels[21].
The elimination half -life of ketamine is 100-180 minutes. However, in the current study the time for first analgesic dose after ketamine wound infiltration was 7.7 ± 2.41 h and FLACC
scores were significantly lower 4 to12 hours postoperatively compared to bupivacaine. This prolonged effect can be explained by ketamine’s anti-inflammatory effects as well as its ability to decrease acute opiate tolerance and central hypersensitization of nociceptors. Moreover, the active metabolite, nor-ketamine also contributes to the extended analgesic effects of ketamine[22].
The results of the study carried by Oham et al[23]. revealed that subcutaneous ketamine prolonged the analgesic effect of bupivacaine after unilateral herniotomy in children.
According to Mohamed et al.[8], combining ketamine with bupivacaine for wound infiltration in patients undergoing total abdominal hysterectomy provided better perioperative analgesia in addition to attenuating the stress response to surgery.
Moreover, addition of ketamine to levobupivacaine prolonged the duration of postoperative analgesia, and decreases 24 h opioid consumption when used for wound infiltration in cesarean sections[24].
When compared directly to bupivacaine for surgical site infiltration in open cholecystectomy and pyelolithotomy, ketamine had a comparable effect to bupivacaine but with a longer duration of action[25]. Similarly, wound infiltration with ketamine showed a longer time for rescue analgesia compared to bupivacaine in cleft palate surgeries[26].
The dose of ketamine in the aforementioned studies ranged from 0.5 – 2 mg/kg. However, according to Sarhan et al.[27], pre-incisional tonsillar infiltration doses as low as 0.25 yielded satisfactory pain relief with minimal side effects.
The role of ketamine as a sole agent for wound infiltration has been assessed in previous studies. Pre-incisional ketamine at a dose of 2 mg/kg successfully decreased pain scores after open cholecystectomy compared to placebo[28]. Surprisingly, in the study carried by Khajavi et al.[29] to compare ketamine, tramadol, tramadol + ketamine and placebo (16 patients in each group) for incisional infiltration in renal surgery, ketamine was comparable to placebo. This may be related to small sample size and the smaller dose of ketamine used in the study (0.5 mg/kg).
It should be noted that wound infiltration (WI) with local anesthesia is considered as part of ‘basic-level’ multimodal postoperative analgesia according to the European Society of Pediatric Anesthesia (ESPA) pain management ladder[30]. Despite improvements in the effectiveness and duration of the provided analgesia owing to the use of adjuvants, there is no evidence that single-shot WI can be used as an alternative to more advanced techniques as ultrasound guided abdominal blocks and epidural analgesia. Nevertheless, single shot WI remains an attractive choice in low resource settings.
An important modification of the WI technique is the use of catheters to provide continuous wound infiltration (CWI). According to a previous meta-analysis, CWI was comparable to epidural analgesia as regards pain scores at rest 24 hours after abdominal surgery with less incidence of urinary retention. [31] Another systematic review and meta-analysis concluded that pre-peritoneal catheters were superior to subcutaneous catheters and can be used as an alternative to epidural analgesia with less incidence of hypotension and better patient satisfaction[32].
The nociceptive system is not fully developed in early years of life. Repeated painful experiences may alter brain plasticity and retard neurodevelopment. Hence, in children, it is crucial to implement multimodal strategies that block most of the steps of nociception. Accordingly, WI should always be used as part of a ‘multimodal approach’ with pre-incisional infiltration being superior to post-incisional techniques as blocking early steps of nociception prevents propagation of pain impulses to the cerebral cortex, thereby minimizing the potential impact on neuro- plasticity[33].
Apart from incisional infiltration, both ketamine and dexamethasone have been compared as adjuvants to local anesthetics in peripheral nerve blocks with discrepant results. The analgesic effect of both drugs was comparable but superior to lidocaine alone when used in transthoracic paravertebral and combined femoral- sciatic nerve blocks[34]-[35]. Time for first analgesic request was longer with dexamethasone vs ketamine when combined with bupivacaine in inter-scalene block for arthroscopic shoulder surgeries[36]. Similarly, when combined with lidocaine, dexamethasone was superior to ketamine in terms of increasing duration of sensory and motor axillary blocks in patients undergoing hand and forearm soft tissue surgeries[37].
-
Limitations and future aspects
The main limitation of this study is that the type of abdominal surgery in the children included in the study was not unified which may have led to a variation in postoperative pain intensity. Another limitation is that the inclusion of a wide range of age groups may have led to variations in the pharmacokinetics and pharmacodynamics of the analgesic drugs used, potentially impacting analgesia times.
Further multi-centric studies with larger sample sizes are still needed to support the results of the current study. Investigating other outcomes such as long-term analgesic effects, onset of mobilization and duration of hospital stay should also be considered. Moreover, visceral pain is not covered by WI techniques, and combining WI with other regional anesthesia techniques to cover all the steps of nociception should be put into consideration
-
Conclusion
Combining either ketamine or dexamethasone with bupivacaine for incisional infiltration prolonged the duration of analgesia after pediatric abdominal operations.
-
References
1. Lee J, Jo YY. Attention to postoperative pain control in children. Korean J Anesthesiol .2014, 66(3), 183-188. https://doi.org/10.4097/kjae.2014.66.3.183
2. Stamenkovic DM, Bezmarevic M, Bojic S, et al. Updates on Wound Infiltration Use for Postoperative Pain Management: A Narrative Review. J Clin Med. 2021, 10 (20), 4659. Published 2021 Oct 11. https://doi.org/10.3390/jcm10204659
3. Whiteman A, Bajaj S, Hasan M. Novel techniques of local anaesthetic infiltration. BJA Educ.2011, 11(5), 167-171, https://doi.org/10.1093/bjaceaccp/mkr026
4. Swain A, Nag DS, Sahu S, Samaddar DP. Adjuvants to local anesthetics: Current understanding and future trends. World J Clin Cases .2017, 5(8 ), 307-323. https://doi.org/10.12998/wjcc.v5.i8.307
5. Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016, 37(7), 865-872. https://doi.org/10.1038/aps.2016.5
6. Gordon KG, Choi S, Rodseth RN. The role of dexamethasone in peripheral and neuraxial nerve blocks for the management of acute pain. South African J. Anaes. 2016, 22(6), 163-169. https://doi.org/10.1080/22201181.2016.1251063
7. Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs.1997, 23(3), 293-297.
8. Mohamed SA, Sayed DM, El Sherif FA, Abd El-Rahman AM. Effect of local wound infiltration with ketamine versus dexmedetomidine on postoperative pain and stress after abdominal hysterectomy, a randomized trial. Eur J Pain. 2018, 22(5), 951-960. https://doi.org/10.1002/ejp.1181
9. Subhedar R, Shelke S, Patil R, SubhedarA, Ambre V. Does dexamethasone improve postoperative day care surgery outcome when used as additive to local infiltration anesthesia: A randomized case control study. Indian J Clin Anaesth. 2016, 3(1), 74-79. https://doi.org/10.5958/2394-4994.2016.00016.0
10. Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta An- aesthesiol Scand. 1990, 34(5), 335-338. https://doi.org/10.1111/j.1399-6576.1990.tb03097.x
11. Gordon SM, Chuang BP, Wang XM, et al. The differential effects of bupivacaine and lidocaine on prostaglandin E2 release, cyclooxygenase gene expression and pain in a clinical pain model. Anesth Analg. 2008, 106(1), 321-327. https://doi.org/10.1213/01.ane.0000296474.79437.23
12. Jia Y, Zhao C, Ren H, Wang T, Luo F. Pre-emptive scalp infiltration with dexamethasone plus ropivacaine for postoperative pain after craniotomy: a protocol for a prospective, randomized controlled trial. J Pain Res. 2019, 12:1709-1719 . https://doi.org/10.2147/JPR.S190679
13. Evaristo-Mendez G, De Alba-Garcia GEG, Sahagun-Flores JE, Ventura-Sauceda FA, Mendez-Ibarra JU, Sepulveda-Castro RR. Analgesic efficacy of the incisional infiltration of ropivacaine vs ropivacaine with dexamethasone in the elective laparoscopic cholecystectomy. Cir Cir. 2013, 81(5):383-93.
14. Pawanna P , Chanthasenanont A, Pongrojpaw D , Limpivest U , Pattaraarchachai JA , Suwannarurk K. Comparison of wound infiltration of lidocaine with and without dexamethasone on episiotomy wound for pain management: A randomized doubleblinded controlled trial .J Med Assoc Thai. 2020, 103(3):70.
15. Holte K, Werner MU, Lacouture PG, Kehlet H. Dexamethasone prolongs local analgesia after subcutaneous infiltration of bupiva- caine microcapsules in human volunteers. Anesthesiology. 2002, 96(6):1331-5. https://doi.org/10.1097/00000542-200206000-00011
16. Rao TN, Goswami D, Roychoudhury A, Bhutia O, Baidya DK, Trikha A. Efficacy of local anesthetic wound infiltration in temporomandibular joint ankylosis surgery for control of postoperative pain: A prospective, randomized controlled, and doubleblinded trial. J Oral Maxillofac Surg. 2021, 79(3):559.e1-559.e11. https://doi.org/10.1016/j.joms.2020.10.034
17. Basuni AS, Ezz HA, Albirmawy OA. Preoperative peritonsillar infiltration of dexamethasone and levobupivacaine reduces pediatric post-tonsillectomy pain: a double-blind prospective randomized clinical trial. J Anesth. 2013, 27(6), 844-849. https://doi.org/10.1007/s00540-013-1638-0
18. Montazeri K, Okhovat A, Honarmand A, Safavi MR, Ashrafy L. Pre-incisional infiltration of tonsils with dexamethasone dose not reduce posttonsillectomy vomiting and pain in children. Saudi J Anaesth. 2009, 3(2): 53-56. https://doi.org/10.4103/1658-354X.57874
19. Veneziano G, Martin DP, Beltran R, et al. Dexamethasone as an Adjuvant to Femoral Nerve Block in Children and Adolescents Undergoing Knee Arthroscopy: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Reg Anesth Pain Med. 2018, 43(4), 438-444. https://doi.org/10.1097/AAP.0000000000000739
20. Chong MA, Szoke DJ, Berbenetz NM, Lin C. Dexamethasone as an Adjuvant for Caudal Blockade in Pediatric Surgical Patients: A Systematic Review and Meta-analysis. Anesth Analg. 2018, 127(2), 520-528. https://doi.org/10.1213/ANE.0000000000003346
21. Pai A, Heining M. Ketamine. Continuing Education in Anaesthesia Critical Care & Pain.2007, 7(2):59-63. https://doi.org/10.1093/bjaceaccp/mkm008
22. Sigtermans MJ, Van Hilten JJ, Bauer MC, et al. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009, 145(3): 304 -311. https://doi.org/10.1016/j.pain.2009.06.023
23. Oham A, Ekwere I, Tobi K. Subcutaneous ketamine prolongs the analgesic effect of local infiltration of plain Bupivacaine in children undergoing inguinal herniotomy. Afr Health Sci. 2020, 20(2): 806-814. https://doi.org/10.4314/ahs.v20i2.34
24. Kaler P, Verma I, Grewal A, Taneja A, Sood D. Comparison of levobupivacaine alone versus levobupivacaine with ketamine in subcutaneous infiltration for postoperative analgesia in lower segment cesarean section. J Obstet Anaesth Crit Care. 2019, 9:60-4. https://doi.org/10.4103/joacc.JOACC_25_19
25. Bhola S, Aske R. Effects of preincisional analgesia with surgical site infiltration with 0.25% bupivacaine or with ketamine in patients undergoing open cholecystectomy and pyelolithotomy surgery under general anaesthesia. Int. J. Contemp. Med. 2019, 6 (1):A8-A11. https://doi.org/10.21276/ijcmr.2019.6.1.30
26. Jha AK, Bhardwaj N, Yaddanapudi S, Sharma, RK, Mahajan JK. A randomized study of surgical site infiltration with bupivacaine or ketamine for pain relief in children following cleft palate repair. Paediatr Anaesth. 2013, 23: 401- 406. https://doi.org/10.1111/pan.12124
27. Sarhan, N, Fatahalla M, Ahmed M, Osman H. Analgesic Effect of Pre-Incisional Peritonsillar Infiltration of Ketamine for Post-Tonsillectomy Pain in Children. Int J Otorhinolaryngol Head Neck Surg. 2018, 7:317-329. https://doi.org/10.4236/ijohns.2018.76032
28. Safavi M, Honarmand A, Nematollahy Z. Pre-incisional analgesia with intravenous or subcutaneous infiltration of ketamine reduces postoperative pain in patients after open cholecystectomy: A randomized, double-blind, placebo-controlled study. Pain medicine. 2011, 12(9):1418-1426. https://doi.org/10.1111/j.1526-4637.2011.01205.x
29. Khajavi MR, Navardi M, Shariat Moharari R, et al. Combined Ketamine-Tramadol Subcutaneous Wound Infiltration for Multimodal Postoperative Analgesia: A Double-Blinded, Randomized Controlled Trial after Renal Surgery. Anesth Pain Med. 2016, 6(5), e37778. https://doi.org/10.5812/aapm.37778
30. Vittinghof M et al. Postoperative pain management in children: Guidance from the pain committee of the European Society of Pediatric Anaesthesiology (ESPA Pain Management Ladder Initiative). Paediatr Anesthes. 2018, 28(6): 493-506. https://doi.org/10.1111/pan.13373
31. Ventham NT, Hughes M, O’Neill S, Johns N, Brady RR, Wigmore SJ. Systematic review and meta-analysis of continuous local anesthetic wound infiltration versus epidural analgesia for postoperative pain following abdominal surgery. Br J Surg.2013, 100(10): 1280-1289. https://doi.org/10.1002/bjs.9204
32. Mungroop TH, Veelo DP, Busch OR. Continuous wound infiltration versus epidural analgesia after hepato-pancreato-biliary surgery (POP-UP): a randomized controlled, open-label, non-in- feriority trial. Lancet Gastroenterol hepatol. 2016, 1(2):105-113. https://doi.org/10.1016/S2468-1253(16)30012-7
33. Loizzo A, Loizzo S, Capasso A.Neurobiology of pain in children: An Overview. Open Biochem J. 2009, 3: 18-25. https://doi.org/10.2174/1874091X00903010018
34. El Mourad MB, Amer AF. Effects of adding dexamethasone or ketamine to bupivacaine for ultrasound-guided thoracic paravertebral block in patients undergoing modified radical mastectomy: A prospective randomized controlled study. Indian J Anaesth. 2018, 62(4), 285-291. https://doi.org/10.4103/ija.IJA_791_17
35. Zekry J, Sayed N, Hasanin A, Mostafa M. Ketamine versus Dexamethasone as an Adjuvant to Local Anesthetics in Combined Sciatic-Femoral Nerve Block for Below Knee Surgeries. MJMR .2015, 26 (1): 32-38.
36. Elbahrawy K, El-Deeb A. Dexamethasone Versus Ketamine in the Interscalene Block in Patients Undergoing Arthroscopic Shoulder Surgery: A Randomized Double-Blinded Study. Asian J Anesthe- siol. 2018, 56(4), 136-142. https://doi.org/10.6859/aja.201812_56(4).0003
37. Zaman B, Hojjati Ashrafi S, Seyed Siamdoust S, Hassani V, Mohamad Taheri S, Noorizad S. The Effect of Ketamine and Dexamethasone in Combination with Lidocaine on the Onset and Duration of Axillary Block in Hand and Forearm Soft Tissue Surgery. Anesth Pain Med. 2017, 7(5), e15570. https://doi.org/10.5812/aapm.15570

 ORCID
ORCID