Shibu Sasidharan MD, DNB, MNAMS 1
Recibido: 07-01-2021
Aceptado: 27-01-2021
©2021 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 50 Núm. 3 pp. 439-454|https://doi.org/10.25237/revchilanestv50n03-04
PDF|ePub|RIS
Covid-19: Desafíos, recomendaciones, revisión e investigación
Abstract
Though physicians and care providers are familiar with the management of ARDS, however, when it occurs as a sequale of COVID-19, COVID-19 ARDS has different features and there remains uncertainty on the consensus of management. To answer this question on how it compares and contrasts with ARDS from other causes, we deliver a review of the published literature and our own clinical experience from managing patients with COVID-19 ARDS in DR Congo and India. A PubMed search was conducted on 05-7-2020 using the systematic review filter to identify articles that were published using MeSH terms COVID-19 and ARDS. Systematic reviews or meta-analyses were selected from a systematic search for literature containing diagnostic, prognostic and management strategies in MEDLINE/PubMed. Those were compared and reviewed to the existing practices by the various treating specialists and recommendations were made. Specifically, we discuss the COVID-19 ARDS, its risk factors and pathophysiology, lab diagnosis, radiological findings, rational of recommendation of drugs proposed so far, oxygenation and ventilation strategies and the psychological ramifications of the disease. Because of the high mortality in mechanically ventilated patients, the above recommendations and findings direct the potential for improvement in the management of patients with COVID-19 ARDS.
Resumen
Aunque los médicos y los proveedores de atención están familiarizados con el manejo de ARDS, cuando ocurre una complicación de COVID-19, existe incertidumbre sobre el manejo y curso que va a seguir. Para responder a esta pregunta sobre cómo se compara y contrasta con el SDRA por otras causas, entregamos una revisión de la literatura publicada y nuestra propia experiencia clínica en el manejo de pacientes con SDRA COVID-19 en la República Democrática del Congo e India. Se realizó una búsqueda en PubMed el 05 de julio de 2020 utilizando el método sistemático con filtro de revisión para identificar artículos que se publicaron utilizando términos MeSH COVID-19 y SDRA. Se seleccionaron revisiones sistemáticas o metanálisis de una búsqueda sistemática de literatura que contenga diagnóstico, pronóstico y manejo estrategias en MEDLINE / PubMed. Aquellos fueron comparados y revisados para las prácticas existentes por los diversos especialistas en tratamiento y recomendaciones que fueron hechos. Específicamente, discutimos el ARDS COVID-19, sus factores de riesgo, fisiopatología, diagnóstico de laboratorio, hallazgos radiológicos, racionalidad de recomendación de los fármacos propuestos hasta el momento, las estrategias de oxigenación y ventilación y las complicaciones psicológicas de la enfermedad. Debido a la alta mortalidad de los paciente en ventilación mecánica las recomendaciones y los hallazgos anteriores se dirigen a la potencial de mejora en el manejo de pacientes con COVID-19.
-
Introduction
The coronavirus disease 2019 (COVID-19) is an acute infectious disease caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The World Health Organization has labelled COVID-19 as a global infectious disease pandemic. COVID-19 is the third major outbreak caused by coronavirus in this century, with the earlier ones being severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS). Physicians and care providers are familiar with the management of ARDS, however, when it occurs as a sequelae of COVID-19, ‘COVID-19 ARDS’ has different features and there remains uncertainty on the consensus of management. To answer this question on how it compares and contrasts with ARDS from other causes, we deliver a review of the published literature (Pubmed Search on 05-7-2020, using MeSH terms COVID-19 and ARDS) and our own clinical experience from managing patients with COVID-19 ARDS in DR Congo and India. This information will provide an insight to the challenges faced by the ICU teams and give a comprehensive multi-speciality recommendations for circumnavigating these difficulties.
-
History
The World Health Organization (WHO) on 31 December 2019, formally notified about a cluster of cases of pneumonia in Wuhan City, – central China. By 05 January 2020, 59 cases were known and none had been fatal[1]. Ten days later, there were 282 confirmed cases, of which four were in Japan, South Korea and Thailand[2]. By then, there had been six deaths in Wuhan, 51 people were severely ill and 12 were critical. The responsible pathogen was isolated on 7 January and its genome was shared on 12 January[3]. The causative organism of the severe acute respiratory syndrome, now named as COVID-19, was a novel coronavirus, SARS-CoV-2. Today history is continuously being rewritten, and as of 08 July 2020 – there are 12 M confirmed cases and 548 K deaths worldwide. 168,957 new cases of COVID-19 worldwide were being confirmed daily and the death rate was over 4147 per day[4]. These numbers are conceivably an underestimate of the actual infected and dead due to restrictions of surveillance and testing.
-
Clinical features and classification
The patients are classified vide, WHO, and CCDC guidelines into these categories on the basis of their symptoms[5].
a) Mild Illness: Fever, cough, myalgia, headache, nausea, anorexia, anosmia, etc. with temperature ranging upto 38° C but no shortness of breath. Treatment is symptomatic. Patient managed on a domiciliary basis, by self monitoring and quarantine, and reports to the hospital only if any danger signs evolve[6].
b) Moderate Illness: Patients present with higher grade fever, shortness of breath with tachypnoea (respiratory rate 20-25/min), and features of early pneumonia on chest X-ray but SpO2 > 93%. These patients may warrant management in the isolation ward at the hospital.
c) Severe Illness: Characterized by worsening dyspnoea (respiratory rate > 30/min, SpO2 < 93%), fatigue, fever, impaired mental acuity besides lymphopenia, and chest X ray revealing features of bilateral pneumonia warranting treatment at Severe Acute Respiratory Illness (SARI) centre with supplemental oxygen, intravenous access, pulse oximetry, cardiac monitoring besides possible resuscitation/intubation eventually.
d) Critical Illness: Severe respiratory distress and hypoxia despite supplemental oxygen, obtundation or loss of consciousness, evidence of multi-organ dysfunction requiring intensive care including intubation and mechanical ventilation.
-
Definition of ARDS
COVID-19 ARDS is diagnosed when someone with a confirmed COVID-19 infection meets the Berlin 2012 ARDS diagnostic criteria[7], which include:
i) acute hypoxaemic respiratory failure;
ii) presentation within 1 week of worsening respiratory symptoms;
iii) bilateral airspace disease on chest x-ray, computed tomography (CT) or ultrasound that is not fully explained by effusions, lobar or lung collapse, or nodules; and
iv) cardiac failure is not the primary cause of acute hypoxaemic respiratory failure.
-
Lung & diagnostic pathology
SARS-CoV-2 infection can be confirmed by positive detection of viral RNA in nasopharyngeal secretions using a specific PCR test (RT-PCR).
Protocol for laboratory testing[8] is as.
In the lung, SARS-CoV-2 causes acute diffuse alveolar damage, pneumocyte hyperplasia, and interstitial pneumonia.
In the acute stage of ARDS, there is diffuse alveolar damage in the lung along with formation of hyaline membrane in the alveoli which is followed sequentially by interstitial widening oedema and later proliferation of fibroblasts in the organizing stage[9]. COVID-19 ARDS causes the typical ARDS pathological changes of diffuse alveolar damage in the lung[10]. During the illness, lung fibrosis appears in the long term.
Coagulation dysfunction is common in COVID-19 (detected by raised D-dimer levels). Fatal cases have shown diffuse microvascular thrombosis, suggesting a thrombotic microangiopathy, and evidence of thrombotic disseminated intravascular coagulation[11]. This explains the atypical manifestations seen in the lung, like dilated pulmonary vessels on the CT Chest, and episodes of pleuritic pain. Vascular enlargement is not seen in typical ARDS, but seen in most cases of COVID-19 ARDS[12].
Microscopy: Microscopic involvement included exudative and proliferative phases of diffuse alveolar damage. Electron microscopy revealed that viral particles were predominantly located in the pneumocytes. The predominant pattern of lung lesions in patients with COVID-19 is diffuse alveolar damage, as described in patients infected with SARS and MERS corona viruses. Hyaline membrane formation and pneumocyte atypical hyperplasia are frequent. The presence of platelet-fibrin thrombi in small arterial vessels is consistent with coagulopathy, which appears to be common in patients with COVID-19 and should be one of the main targets of therapy[13].
Biomarkers: Recent studies have suggested that in addition to direct viral damage, uncontrolled inflammation contributes to disease severity in COVID-19. Consistent with this hypothesis, high levels of inflammatory markers, including CRP, ferritin, D-dimer, high neutrophil-to-lymphocyte ratio, increased levels of inflammatory cytokines and chemokines have been observed in patients with severe disease. Pathogenic inflammation, also referred to as cytokine storm, shares similarities with SARS-CoV and MERS-CoV10. Inflammatory cytokines IL-6, IL-8, TNF-, and IL-1 could help predict the course and outcome of disease in COVID-19. A high IL-6 predicted a 227% increase in chances of death, and TNF- reduced the chances of survival by 150%. It has been found that when IL-6 and TNF- are high at the time of admission, the patient is likely to have severe disease and reduced survival, irrespective of the use of other clinical and laboratory findings[14].
Procalcitonin (PCT) has emerged as a crucial biomarker for the severity and prognosis of COVID-19 infection. Italian researchers have reported that the risk of severe SARS-CoV-2 infection was nearly five times higher in COVID-19 patients with raised PCT levels. A retrospective, multi-center study of 191 confirmed COVID-19 cases in Wuhan, China, reported that three indicators-higher Sequential Organ Failure Assessment (SOFA) score, a D-dimer ≥ 1 µg/L, and advanced age-signify higher mortality risk. These markers could help identify patients in the early stages of COVID-19 with a poor prognosis.
Laboratory : Peripheral white blood cell (WBC) count, neutrophil-to-lymphocyte ratio (NLR), derived NLR ratio ((d-NLR), neutrophil count divided by the result of WBC count minus neutrophil count), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) are indicators of the systematic inflammatory response that are widely investigated as useful predictors for the prognosis of viral pneumonia. WBC count, NLR, LMR, PLR, CRP, and d-NLR of severe patients were significantly higher than those of non-severe patients. The optimal threshold at 3.3 for NLR showed a superior prognostic possibility of clinical symptoms to change from mild to severe, which had the highest of sensitivity and specificity. When age ≥ 49.5 years and NLR ≥ 3.3, 46.1% of the COVID-19 patients with mild disease became severe in a mean time of 6.3 days. Therefore, these patients must be closely attended to by clinicians. By contrast, when age < 49.5 years and NLR < 3.3, COVID-19 patients with mild disease were cured and discharged in approximately 13.5 days[15].
-
Determinants of adverse outcomes
COVID-19 ARDS appears to have significantly worse outcomes compared to ARDS from other causes.
Risk factors for poor outcomes include advanced age; presence of comorbidities such as hypertension, cardiovascular disease, and diabetes mellitus; lower lymphocyte counts; kidney injury; and raised D-dimer levels[18].
-
Radiodiagnosis and imaging in covid-19 ards
Imaging assists in establishing diagnosis, triage and providing management guiding actionable results[19]. The radiological armamentarium available for COVID includes routine chest radiograph, Computed Tomography (CT), and point of care thoracic ultrasonography (POCUS)[20].
Chest radiography (CXR) is sensitive only when patients present late or with advanced symptoms. CXR findings include patchy peripheral consolidation or ground glass opacities (GGO) with predilection for lower and middle lobes. The consolidation is bilateral in 75% of patients and unilateral in 25%[21]. Occasional nodules, perihilar consolidation, and prominence of perihilar vasculature are also noted[22].
Non-contrast high resolution continuous helical CT scan of the thorax is the preferred protocol for evaluating COVID-19 patients. However, contrast may be administered in select cases to exclude other causes like pulmonary thromboembolism[23]. GGO with peripheral and lower lobe predilection are the most common findings. In addition, crazy paving (GGO with thickened interlobular and intralobular septa), vascular distension in region of GGO may be seen early in the disease[19],[23]. Later, the imaging appearance progresses to architectural distortion, subpleural bands, fibrosis, and traction bronchiectasis. Consolidation may superimpose on the GGO later in the disease and in older and high-risk individuals. Pleural effusion, pericardial effusion, lymphadenopathy, and pneumothorax are also uncommonly seen[19],[23].
COVID-19 can initially present as a subpleural disease. Therefore the accuracy of POCUS as screening tool is limited. It has significant value, however, in monitoring the progress of critically COVID-19 patients in ICU. The recommendations of Fleischner Society on the role of chest imaging in COVID patients are as under[19].
-
Medical management of sars-coV-2 infection
In view of the lack of availability of approved specific drug therapy for SARS-CoV-2 treatment is essentially supportive and symptomatic. The initial step involves triage of patients of SARS-CoV-2 into mild, moderate, severe, and critical categories depending upon the severity of clinical presentation vide WHO, and CCDC guidelines[24].
Patients with worsening hypoxia require management in hospital with supplemental oxygen by either high flow nasal cannula (HFNC) or non-invasive ventilation. Intubation and mechanical ventilation are indicated in patients having severe illness. They may also require concomitant intensive care management of multiorgan dysfunction by a multidisciplinary team of treating specialists. The treatment of severe COVID-19 illness includes aggressive treatment of complications, prophylaxis for secondary infection, thrombotic events and organ function support based on treatment of underlying disease[25]. ICU practices that prevent ARDS or aid in early recognition and effective treatment of the events leading to ARDS, like lung-protective ventilation and conservative fluid management, remain essential elements to achieve desired improved outcomes.
As there is no approved specific pharmacotherapy for COVID-19, various drugs have been tried by treating doctors across the world with variable results. Currently, research trials are underway to find a definite cure, but there is no consensus on a specific drug being effective in curing SARS-CoV-2 infection. Experimental and repurposed therapies that stand unsupported by strong evidence are to be strongly discouraged.
-
Treatments under evaluation for covid-19
a) Hydroxychloroquine: Based on experience with earlier viral illnesses, HCQ was proposed to be likely effective therapy for COVID-19 besides prophylaxis. In an, observational study by Joshua et al. involving 1,376 patients with COVID-19 admitted to the hospital, hydroxychloroquine administration was not associated with either a greatly lowered or an increased risk of the composite end point of intubation or death[26]. It has now been stopped due to lack of efficacy. WHO guidelines recommend against prescribing HCQ for prophylaxis (both post and pre-exposure) in individuals with confirmed or suspected exposure to SARS-CoV-2.
b) Hydroxychloroquine plus azithromycin: Combination therapy was initially attempted to treat COVID-19, however, subsequently discontinued due to cardiac arrhythmias secondary to increased QT interval resulting in fatality in a few.
c) Lopinavir-ritonavir: These anti-retroviral drugs, initially considered promising in SARS-CoV-2 infection failed in expected outcomes. WHO accepted the recommendation from Solidarity Trial’s International Steering Committee vide press release dated 04 July 2020 to discontinue lopinavir-ritonavir arm of the trial due to evidence of little or no reduction in mortality of hospitalized COVID-19 patients when compared to standard of care.
d) Favipiravir: This selective RNA polymerase inhibitor, under study in various trials around the world, inhibits viral replication. Two clinical trials (Japan, USA) and a phase-3 clinical trial in India using favipiravir combined with another antiviral agent, Umifenovir are ongoing[27]. Results are awaited.
e) Remdesivir: A nucleotide analogue prodrug that is intracellularly metabolized to an analogue of adenosine triphosphate, that inhibits viral RNA polymerases has shown in-vitro activity against SARS-CoV-2. The first published report with a group of patients receiving remdesivir in a compassionate-use programme, described clinical improvement in 36 of 53 hospitalized patients (68%) with severe COVID-19[27]. US FDA issued an EUA of remdesivir to allow its emergency use for severe COVID-19 (confirmed or suspected) in hospitalized patients[28],[29]. Currently, several phase-3 clinical trials are evaluating it for treatment of moderate and severe COVID-19.
f) Dexamethasone: Practise of using dexamethasone varied widely across the world with many treatment guidelines having conflicting reports on use of corticosteroids in COVID-19 illness[30] but in China they were being used in severe cases[31]. However, the RECOVERY trial (with over 11,500 patients enrolled from over 175 NHS hospitals in the UK) provided clear evidence that dexamethasone 6mg per day for up to 10 days reduces 28-day mortality in COVID-19 patients receiving invasive mechanical ventilation by one third, and by one fifth in patients receiving oxygen without invasive mechanical ventilation. No benefit was demonstrated in hospitalized COVID-19 patients who were not receiving respiratory support and results were consistent with possible harm in this group[32].
g) Convalescent plasma: Convalescent plasma, collected from donors having recovered from recent COVID-19 infection, contains anti-SARS-CoV-2 virus antibodies that can be used to treat other COVID-19 patients. Data from a study in USA involving 20,000 patients transfused with COVID-19 convalescent plasma demonstrate that its use is safe and carries no excess risk of complications and supports the premise that administration of the same early during illness is likely to reduce mortality[33]. Another study by Liu et al showed that convalescent plasma transfusion improved survival in non-intubated patients but not in intubated patients[34]. The FDA states that it is important to determine its safety and efficacy via clinical trials before routinely administering convalescent plasma to patients with COVID-19.
h) Interleukin-6 (IL-6) inhibitors: Interleukin-6 is a pleiotropic pro-inflammatory cytokine produced by various cell types including lymphocytes, monocytes, and fibroblasts. SARS-CoV-2 virus induces IL-6 production from bronchial epithelial cells causing inflammation. Various IL-6 inhibitors (like sarilumab, tocilizumab) are under evaluation for their efficacy in management of COVID-19. However, presently there is inconclusive data to recommend for or against the use of IL-6 inhibitors[35].
j) Nitric Oxide: Potential role of inhaled nitric oxide (iNO) in preventing progression of disease in those with severe ARDS is under evaluation[36]. Routine use of iNO in patients with COVID-19 pneumonia is not recommended and the trial is recommended only in mechanically ventilated patients with severe ARDS and hypoxemia despite other rescue strategies[37]. Studies are ongoing to evaluate for the efficacy and safety of iNO in SARS-CoV-2 patients requiring supplemental oxygen before the disease progresses to necessitating mechanical ventilatory support[38].
k) COVID-19 Vaccine: As per WHO, there are 19 vaccine trials in clinical evaluation around the world currently. Various countries have given permission to start clinical trials for vaccines being developed by them, e.g., India (Covaxin by Bharat Biotech, ZyCov-D by Zydus Cadila), China (a vaccine by Sinovac in partnership with Brazilian vaccine producer Instituto Butantan, another vaccine by Sinopharm), AstraZeneca’s experimental COVID-19 vaccine developed by researchers at University of Oxford besides a vaccine in Nigeria (based on study funded by Trinity Immunodeficient Labs and Helix Biogen Consult). However, currently no vaccine is available for commercial use to stop COVID-19 pandemic.
-
Oxygenation & ventilation for covid-19 ards patients
COVID-19 ARDS follows an anticipated time course, with a median time to intubation of 8-10 days after symptom onset[39]. It is therefore imperative to constantly monitor patients for the development of ARDS as the day of infection progresses. The primary strategy for COVID-19 patients is supportive care, which includes oxygen therapy for hypoxemic patients. Oxygen therapy is instituted if respiratory rate is of 30 breaths/min or above and/or SpO2 of 93% on breathing air.
-
Non invasive modes
High-flow oxygen therapy (HFNO) should be started if there is a respiratory failure and mild-moderate ARDS. HFNO is used as first-line treatment, followed by Non-invasive ventilation (NIV) in ARDS[39]. However, NIV is not recommended for patients with failed HFNO. NIV provides benefit via PEEP, to patients with mild-moderate ARDS by reducing the respiratory load and intubation rate but it can cause significant aerosol generation.
High-flow nasal cannula (HFNC) for HFNO is effective in improving oxygenation, but due to reports of high amount of aerosol dispersion it was not recommended initially. However further studies in patients with acute hypoxemic respiratory failure, HFNC was proven to avoid intubation compared to conventional oxygen devices, and the scientific evidence of generation and dispersion of bio-aerosols via HFNC showed a similar risk to standard oxygen masks. HFNC prong with a surgical mask on the patient’s face is thus a reasonable modality to benefit hypoxaemic COVID-19 patients and avoid intubation[40].
-
Intubation and invasive mechanical ventilation of covid-19 ards patients
Mechanical ventilation of COVID-19 patients with ARDS is really a challenging task as these patients usually have non homogenous lung pathology. This requires a targeted lung-protective ventilation strategy to improve the outcome.
The indications for mechanical ventilation in COVID-19 ARDS [41,[42] are as follows:
1. Acute hypoxic respiratory failure with severe respiratory distress.
2. Worsening hypoxia associated with increased labored breathing.
3. Increased work of breathing associated with use of accessory muscles of respiration.
4. Failure to maintain SpO2 > 90% with > 50 L/min of high flow oxygen with HFNO or with maximal supplemental oxygen.
5. Hypoxia with altered mental status and failure to maintain airway patency.
6. Patient with multi-organ failure, persistent hemodynamic instability requiring vasopressor support, or those with multiple comorbidities like (DM, Cardiovascular disease, hypertension, advanced age, frailty, cancer or chronic respiratory disease).
7. Arterial pH < 7.3 with PaCO2 > 50 mm Hg.
8. PaO2/FiO2 < 200[43].
9. High respiratory rate with persistent thoraco-abdominal asynchrony or paradoxical respiration.
10. Low ROX index[44] (< 4.88) with patient on HFNC.
The indications for intubation and mechanical ventilation in COVID-19 patients are not limited to the above mentioned conditions and it should be at the discretion of the treating physician [45].
-
Precautions & Procedures while intubating COVID-19 patients
Airway management and intubation in COVID-19 patients is an aerosol generating procedure and is associated with increased risk of viral transmission to the health care providers. Hence, a high level of attentiveness and alertness is necessary to prevent infection when intubation is performed. The following points are to be ensured for safety of patients and health care providers[46]:
1. Standard level 3 protection to be donned while performing intubation.
2. Standard monitoring, IV access, instruments, drugs, ventilator, and suction should be pre- checked.
3. Tracheal intubation to be performed by the most experienced anaesthesiologist in an airborne infection isolation room to ensure patient safety and HCW (Health care worker).
4. Limit the number of health care provider in the room/cubicle prior to intubation.
5. Use 3-5 minutes pre-oxygenation with 100% oxygen as these critical patients have poor oxygen reserve.
6. Spontaneous ventilation to be preserved and avoid assisted bag mask ventilation during preoxygenation.
7. RSI (rapid sequence intubation) technique to be used to avoid manual ventilation of the patient’s lungs and the potential aerosolization of the virus from the airways[47].
8. Use both hands to hold the mask to ensure a tight seal using the V-E technique rather than the C-E technique with one hand.
9. Video laryngoscope is preferred for intubation.
10. Airway management should be safe, accurate and should be accomplished within 15-20 seconds.
11. After tracheal intubation, clamp the endotracheal tube (ETT) and inflate the cuff before instituting ventilation[48].
12. Viral and HME filter to be applied between endotracheal tube and circuit.
13. Proper tube placement can be identified by EtCO2 monitoring & visible bilateral chest rise. Avoid auscultation to confirm tube placement.
14. Supraglottic airway devices (SGAD) to be used in CICO (Can`t intubate and can`t oxygenate) situations only and bedside tracheostomy to be performed as early as possible.
-
Ventilatory strategy for COVID-19 ARDS
The most appropriate time to intubate COVID-19 patients is still not clear. However, early and timely institution of mechanical ventilation can be considered if the COVID-19 patient develops moderate to severe ARDS (PaO2/FiO2 < 200) to prevent P- SILI (Patient self-induced lung injury)[43]. Non-intubated spontaneously breathing ARDS patients are at increased risk of P-SILI due to high intake of inhaled tidal volume. Therefore, oesophageal pressure measurement by manometer can be considered in spontaneously breathing, non-intubated patients to decide the time for intubation[49]. The oesophageal pressure between 05 to 10 cm H2O is usually well tolerated. However, if pressure progresses beyond 15 cm H2O, then the risk of P-SILI increases and intubation shouldn’t be delayed. If oesophageal manometry is not available, then change in CVP (central venous pressure) with respiration or clinical assessment of excessive inspiratory effort for increased work of breathing can be considered[50].
Mortality is very high for COVID-19 ARDS patients on mechanical ventilation. Inappropriate ventilatory strategy in ARDS patients can lead to VILI (ventilator induced lung injury), which includes barotrauma (high airway pressure), volutrauma, atelectrauma, biotrauma, myotrauma (diaphragmatic injury) and oxytrauma (oxygen free radicals).
Strategies to promote lung protection in ARDS:
a. Lung protective ventilation[51]
This approach of ventilation in patients with ARDS is based upon several randomized trials and meta-analyses that have reported survival benefit from lung protective ventilation. Initial ventilatory settings for these patients are recommended as below.
b. Role of PEEP in COVID-19 ARDS
There is an ambiguity with respect to the usage of adequate PEEP for COVID-19 ARDS patients. Using higher PEEP (any PEEP > 10 cm H2O) was not recommended based on the heterogenicity of lung involvement in COVID-19 patients with simultaneous existence of severely affected areas with non-affected areas in the lung[52]. However, surviving sepsis campaign guidelines on management of critically ill adults from COVID-19, European intensive and critical care guidelines, advise PEEP > 10 cm H2O for management of ARDS due to SARS-CoV-2.
c. Compliance
In COVID-19 patient’s lung compliance needs to be constantly assessed. If compliance is high or normal with the existence of hypoxemia, it is recommended to use a PEEP of less than 10 cm H2O to avoid over-distention of normal healthy alveoli. However, if compliance is low as seen in ARDS, then it’s advised to use adequate PEEP of just above the lower inflection point on the pressure volume loop on the ventilator to recruit collapsed alveoli, to prevent atelectasis and thereby, improve oxygenation.
Once the initial setting on the ventilator is entered, monitoring of the following parameters is done to ascertain patient progress:
1. Plateau pressure – PPlat should be below 30 cm H2O.
2. Driving pressure is kept below 15 cm H2O. This can be achieved by either decreasing tidal volume (at the risk of development of hypercapnia) or by increasing PEEP, which can cause over-distention of alveoli. Therefore, careful titrations are required.
3. Compliance – Normally the total compliance of both lungs in an adult is about 200 ml/cm H2O. Low compliance is usually found in ARDS patients with stiff lung. There are two types of lung compliance:
a. Static compliance = Tidal volume/(Pplat– PEEP)
Static compliance measures pulmonary compliance when no airflow such as during inspiratory pause and it is slightly higher than dynamic compliance.
b. Dynamic compliance = Tidal volume/(PIP – PEEP)
It represents pulmonary compliance during active inspiration and depends upon peak inspiratory pressure (PIP). PIP depends on airway resistance. COVID-19 pneumonia usually has high compliance (> 40 ml/cm H2O). Therefore, management should be instituted with low PEEP and high tidal volume up to 8-9 ml/kg, if hypercapnia presents.
However, COVID-19 ARDS is to be managed like ARDS with lung protective ventilation by keeping low tidal volume (4-6 ml/kg) and high PEEP.
4. Airway occlusion pressure (PO.1) – The normal value of PO.1 in a spontaneously breathing patient is about 1 cm H2O. However, in mechanically ventilated patients, values above 3.5 cm H2O are associated with increased effort. Keep airway occlusion pressure value in COVID-19 ARDS patients less than 3.5 cm H2O to obtain a ventilatory strategy protective for the lung to prevent it from VILI and diaphragmatic injury (Myotrauma).
d) Target goals of mechanical ventilation
• Target SpO2 = 90%-94%
• PaO2 > 55 mm Hg.
• pH > 7.2
• Fio2 < 0.4
• PaO2/FiO2 > 300 mm Hg.
Subsequent ventilatory setting can be decided by periodic checking of Pplat pressure, driving pressure, compliance, and ABG (pH, oxygenation level). If Pplat pressure > 30 cm H2O, then tidal volume can be decreased to 5 ml/kg or if required, further decreases then tidal volume set to 4 ml/kg of predicted body weight.
e) Adjunctive therapy
The use of adjunctive treatment is relatively less during initial presentation in patients with ARDS, but gradually increase with ARDS severityrecognition, management, and outcomes of patients with the acute respiratory distress syndrome (ARDS).
• Sedation
A combination of multiple agents like (propofol, ketamine, fentanyl, morphine, hydromorphone, dexmedetomidine and midazolam) may be considered for sedation of COVID-19 patients on mechanical ventilator. Usually, COVID-19 patients require high level sedation to ensure patient comfort, alleviate pain, anxiety, avoid ventilator asynchrony & self extubation[53].
• NMBA (neuromuscular blocker agents)
Can be used in boluses in patients with refractory hypoxemia or ventilator asynchrony to facilitate protective and improved lung ventilation. It also causes reduction of high pulmonary inflation pressures (e.g., ARDS), raised intracranial pressure, and metabolic rate (e.g., work of breathing, shivering).
• Recruitment manoeuvres
WHO interim guidelines recommend the use of intermittent recruitment manoeuvres with high PEEP to improve oxygenation in ARDS. However, there are contradicting reports on the use of the same.
• Steroid administration
WHO recommends steroid administration in COVID-19 ARDS patient on mechanical ventilator if they develop septic shock and require increasing dose of vasopressor to maintain MAP > 65 mm Hg in a dose of Inj. Hydrocortisone – 200 mg/day or Prednisolone 75 mg/day.
• Fluid therapy
Conservative or restricted fluid therapy over liberal fluid is advised, as it may worsen oxygenation in mechanically ventilated ARDS patients.
• Management of septic shock
WHO interim guidelines[51] recommend the use of crystalloid intravenous balanced fluids like normal saline, Ringer`s lactate as fluid bolus (01 litre over 30 min or faster) for septic shock to check for fluid responsiveness; and avoid using hypotonic fluids, starch-based solution for resuscitation. If no fluid response occurs OR signs of fluid overload appear like crackles on auscultation, then discontinue the fluid and consider using vasopressors. In vasopressors, norepinephrine is the drug of choice, followed by vasopressin & dobutamine to maintain MAP > 65 mm Hg and preferably be given through central venous line. These vasopressors to be given as per strictly controlled rate decided as per targeted blood pressure to maintain tissue perfusion. However, peripheral lines can be considered in resource-limited settings keeping a close watch for necrosis of skin or extravasation of vasopressors.
f) Prone ventilation
If lung protective ventilation fails to maintain adequate oxygenation and if PaO2/FiO2 < 150 mm Hg with PEEP > 5 and FiO2 > 0.6, then prone ventilation is to be considered. Prone ventilation improves oxygenation and decrease V/Q mismatch, particularly when applied early with other lung-protective strategies. In COVID-19 patients good response to prone positioning may be due to their well-preserved lung compliance compared with patients who develop ARDS from other causes[48],[54]. By optimizing patient selection and treatment protocols, the recently Proning Severe ARDS Patients (PROSEVA) trial demonstrated a significant mortality benefit with prone ventilation.
g) Role of pulmonary vasodilators
The two most commonly used vasodilators in mechanically ventilated patients are inhaled nitric oxide gas (NO) and Epoprostenol, which are administered by continuous inhalation. They can be considered to improve oxygenation even when PaO2/FiO2 < 100 mm Hg despite prone ventilation and if severe hypoxemia is associated with acute pulmonary arterial hypertension[55]. If there is no improvement in the oxygenation after instituting inhaled pulmonary vasodilators, then it should be tapered off without undue delay. The risk of aerosolization and clogging of HME filters is particularly more with epoprostenol, and this remains a concern in COVID-19 patients. That is why inhaled NO is preferred over epoprostenol. In COVID-19 ARDS patients, there is yet no conclusive evidence on the use of pulmonary vasodilators[55].
h) Role of ECMO
Even after prone ventilation, if oxygenation doesn’t improve and hypoxia still persists, then VV-ECMO (veno-venous extracorporeal membrane oxygenation) can be considered. Its use as rescue therapy is considered only in refractory hypoxic respiratory failure[56]. No RCTs or meta-analyses have been conducted for ECMO in COVID-19 patients with ARDS, however, there are reports from China stating its beneficial use. But the process and outcomes have not been mentioned[57].
i) Ventilator weaning and extubation
Special focus needs to be ensured to avoid viral transmission to the health care providers during extubation as it is also an aerosol generating procedure. Since there is a high chance for reintubation in many patients, some physicians like to use cuff leak test criteria along with spontaneous breathing trials (SBT). This is done to assess the readiness for weaning from mechanical ventilation on the assumption that these patients could have developed airway oedema due to prolonged ventilation. Aerosol generation in cuff leak test is similar to extubation, so caution needs to be taken while performing a cuff leak test. SBT without T-piece at lower pressure support (0-3 cm H2O) and along with prior use of steroid to extubation yielded promising results. The following weaning criteria is recommended before extubation:
1. Patient should be conscious, comfortable, and oriented.
2. PaO2/FiO2 > 300 mm Hg with PEEP < 5 cm H2O.
3. Hemodynamically stable and maintaining SpO2 with FiO2 < 0.4.
4. RSBI (Rapid shallow breathing index < 105) – calculated by respiratory rate/tidal volume in litres when the intubated patient is breathing spontaneously.
5. No signs of increased work of breathing or respiratory distress like use of accessory muscles, paradoxical or asynchronous respiration, nasal flaring, profuse diaphoresis, agitation, tachypnoea, tachycardia and cyanosis.
j) Prevention of complications
The prevention of complications associated with mechanical ventilation in COVID-19 patients is important. Some points of focus are:
1. Prevention of VAP[58] by:
a. Spontaneous awakening and spontaneous breathing trials.
b. Elevation of head end.
c. Selective digestive decontamination.
d. Thromboprophylaxis.
e. Oral care without chlorhexidine as some patients develops ARDS due to aspiration of chlorhexidine.
f. Use a new ventilator circuit for each patient.
g. Change HMEs filter when soiled.
2. Reduce pressure sores and ulcers by frequent change of position every 2 hourly.
3. Reduce stress ulcer, gastric bleeding by early enteral feeding and consider PPI or H2 blocker.
4. Reduce ICU related weakness by early mobilisation.
5. Reduce catheter related infection by using sterile aseptic technique while insertion and consider removal when not needed.
6. Reduce the number of days on mechanical ventilation by daily assessment for readiness of extubation through spontaneous breathing trials.
7. Reduce the incidence of venous thromboembolism by use of pharmacological agents or mechanical compression devices.
-
Neuropsychiatric symptoms in covid-19
Long-term outcomes of patients with ARDS are being increasingly recognized as important research targets, as many patients survive ARDS only to have ongoing functional and/or psychological sequelae.
Neuropsychiatric symptoms are atypical presentations of the COVID-19. There is a myriad of symptoms ranging from mild headache and myalgia in majority of cases to life threatening seizures and delirium in patients with severe respiratory compromise (ARDS), especially in patients with underlying co-morbidities.
Neuropsychiatric symptoms are estimated to appear in around 30% of COVID-19 infected patients[59]. Moderate to severe infection can impair executive functions, confusion, and agitation [60].
The neurological complications can be divided into primary neuroinvasion[61] by the corona virus or secondary wave by activated immune and inflammatory mediators. The virus enters the nervous system either directly from the olfactory nerves pathway or is spread via haematogenous route and attaches onto the Angiotensin Converting Enzyme 2 (ACE-2) receptors on the neuronal endothelium[62]. This acute involvement can cause meningitis/encephalitis leading to altered sensorium, delirium, seizures, and/or even coma[63]. It is also hypothesized that direct invasion of medullary neurons could be responsible for severe respiratory failure[64]. Alterations in sensorium and delirium could also be due to hypoxia from respiratory failure, aberrations in coagulation pathways, metabolic imbalances, multi-organ dysfunction, or even iatrogenic (drugs used during mechanical ventilation). Long-term sequelae could be attributed to alterations in immune response and consequent aberrant inflammatory response[65].
-
Delirium
The prevalence of delirium in intubated patients is up to 80%[66] which expectedly upswings in a COVID-19 patient with ARDS.
The risk factors include old age (> 65 yrs), medical co-morbidity, drugs (propofol, opiods, and high-dose benzodiazepines, which are routinely used during mechanical ventilation)[67], hydroxychloroquine[68]. There are certain COVID specific environmental risk factors such as mandatory wearing of personal protective equipment (PPE) which accentuates the anxiety and feeling of vulnerability in an alien environment. The patient is deprived of the reassuring and empathetic look on the doctor’s face[69]. All these risk factors can impair the patient’s perception of the reality and cause disorientation and confusion.
Scales for assessment of delirium
The time tested Confusion Assessment Method for the ICU[70] should be followed routinely. Other useful scales are Intensive Care Delirium Screening Checklist[71] and the Stanford Proxy Test for Delirium[72].
-
Management
A) Non pharmacologic:
1. Ensuring a comfortable ambient light in sync with the diurnal cycle.
2. Ensuring a pain free spell of 6-8 hours of sleep without significant treatment related disruptions.
3. Regular cognitive stimulation and reorientation of the patient to time, place, and person (utilizing AV aids for virtual communication with family members/other familiar people).
4. Encouraging physical mobilization at the earliest.
5. Providing all kinds of possible aids (glasses, hearing aids, mobiles, etc.) to convey a feeling of self-sufficiency and sense of control over the situation.
B) Pharmacologic
1. Sleep cycle:
Melatonin should be used for regularizing sleep-wake cycle in delirium as it has a short half-life, has additional mild anti-inflammatory properties, and does not cause respiratory depression[73]. Suvorexant (Orexin antagonist) has also been used especially in conjunction with Melatonin[74]. Benzodiazepines should be avoided (except in cases of delirium tremens), as cumulative doses run the risk of respiratory depression and may cause paradoxical disinhibition. Zolpidem (2.5-5 mg) is relatively safer in terms of respiratory functioning, but levels are increased in patients taking ritonavir.
2. Acute agitation/Disruptive behavior
Antipsychotic drugs like haloperidol, olanzapine, or quetiapine are found to be beneficial in the management of the agitation. However, monitoring of QTc interval, neurologic side effects (EPS), and sedation are required. The risk of QTc prolongation gets further amplified, given the potential use of COVID-19 specific medications that themselve prolong QTc (hydroxychloroquine, azithromycin), leading to a potentially increased risk of torsades de pointes[75].
a) Haloperidol being a potent dopamine receptor blocker with insignificant anticholinergic and antihistaminic activity (2.5-5 mg) can be used orally or intramuscularly. Intravenous administration should be accompanied by ECG monitoring. Recent research has also shown that haloperidol, due to its effects on sigma receptors, is investigated as a treatment for COVID-19[76],[77].
b) Olanzapine 5-10 mg can also be considered either orally or parenterally. In an acutely disturbed patients, intramuscular (IM) is the preferred route of administration compared to intravenous (IV) route and gluteal IM injections may be preferred over deltoid injections to increase the distance between respiratory secretion/droplet. IM olanzapine has minimal effect on QTc interval and lesser risk for EPS compared to haloperidol.
c) Quetiapine (25-50 mg) can be given orally.
d) Dexmedetomidine is alpha-2 agonist and reduces the release of noradrenaline and helps curtailing restlessness. Clonidine can also be used for the same reason and is more convenient as its available in skin patches form.
e) Valproic acid is known for its neuroprotective[78] potential and can be used to control extreme emotional fluctuations. It also provides prophylaxis against the potentially epileptogenic state by increasing the seizure threshold. However, liver function tests and platelets need to be constantly monitored.
f) In extreme cases not responding to the above measures, only short acting low dose oral benzodiazepines (e.g., lorazepam 1-2 mg) may be considered with close monitoring for respiratory distress and respiratory failure.
g) Mechanical restraint: Mechanical restraint should be used as a last resort for minimum possible time.
3. Mechanical Ventilation
Weaning off mechanical ventilation at times can be associated with acute and severe anxiety that could result in delay in extubation. A very low dose of antipsychotic- Tab Olanzapine 2.5 mg is advisable for anxiolysis.
-
Drug treatment of patients with pre-existing psychiatric illness
Most psychiatric illnesses are remitting and relapsing in nature and generally require long-term prophylaxis. In the absence of a confirmed treatment for management of COVID-19, a multitude of pharmacotherapeautic agents have been tried in the recent past and can have significant drug interactions with psychotropics and can precipitate a relapse of the illness. Hence, it is imperative to be mindful of such interactions.
I. Antipsychotics
Haloperidol, quetiapine, ziprasidone etc. can prolong QTc interval. Hence, chloroquine, hydroxychloroquine, azithromycin, etc. can have a synergistic effect and should be used with caution. Certain protease inhibitors like atazanavir, sequinavir, lopinavir/ritonavir can also cause QTc prolongation. The safer alternatives are lurasidone followed by aripiprazole, olanzapine, and risperidone.
II. Antidepressants
Citalopram, tricyclic antidepressants, and mirtazapine can prolong QTc interval, which might be augmented when combined with hydroxychloroquine, chloroquine. Escitalopram and sertraline are safer in view of lesser drug interactions and side effects.
III. Mood Stabilisers
Non-steroidal anti-inflammatory drugs (NSAIDs) increase lithium levels, which may lead to toxicity. Valproate levels may be reduced with lopinavir/ritonavir.
IV. Sedatives/hypnotics
Longer acting benzodiazepines like diazepam or clonazepam may be avoided. Lorazepam is preferred as it has the least interaction with antiviral drugs and shorter half-life.
-
Conclusión
COVID-19 ARDS is an anticipated severe complication of COVID-19 that requires prompt recognition and comprehensive multi-speciality management. Extensive research and studies are required to address the vital unanswered queries about treatment for COVID-19 ARDS. Because of the high mortality in mechanically ventilated patients, the above recommendations and findings direct the potential for improvement in the management of patients with COVID-19 ARDS.
Declarations: Ethical approval and consent to participate.
Not applicable.
Consent for publication: The authors certify that they have obtained all appropriate permissions for publication.
Availability of data and materials: Yes.
Competing interests: Nil.
Funding: Nil.
Authors’ contributions: All authors have contributed partly or wholly in all or at least 3 of – Study conception and design, Acquisition of data, Analysis and interpretation of data, Drafting of manuscript, Critical revision.
Acknowledgements: Nil.
Authors’ information: As in title page.
References
1. World Health Organization. GCM teleconference – Note for the Records. 10 January 2020. Subject: Pneumonia in Wuhan, China. Available from: – Google Search [Internet].
2. Xie P, Ma W, Tang H, Liu D. Severe COVID-19: A Review of Recent Progress With a Look Toward the Future. Frontiers in Public Health. 2020 May 13;8:189. World Health Organization. Teleconference of the R&D Blueprint GCM. 20 January 2020. Pneumonia of unknown etiology in Wuhan China. Available from: https://www.who.int/blueprint/priority-diseases/key-action/20-01-2020-nfr-gcm.pdf?ua=1
3. World Health Organization. Novel coronavirus (2019‐nCoV). Situation Report – 1. 21 January 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4
4. “WHO Coronavirus Disease (COVID-19) Dashboard.” World Health Organization, World Health Organization,
5. Cascella M, et al. “Features, evaluation and treatment coronavirus (COVID-19).” Statpearls [internet]StatPearls Publishing; 2020.
6. Singhal Tanu. A Review of Coronavirus Disease 2019. The Indian Journal of Pediatrics April 2020 87(4): 281-286. – Google Search [Internet].
7. Berlin 2012 ARDS diagnostic criteria – Google Search [Internet].
8. Clinical management of COVID-19 [Internet].
9. COVID‐19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre‐COVID‐19 ARDS | The Medical Journal of Australia [Internet]. [cited 2020 Jul 9].
10. Xu Z, Shi L,Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; DOI: 10.1016/S22132600(20)30076. – Google Search [Internet].
11. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, Moore HB, Barrett CD. Tissue Plasminogen Activator (tPA) Treatment for COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS): A Case Series. J Thromb
12. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review [- Google Search]. Eur Radiol. 2020 Aug;30(8):4381–9. https://doi.org/10.1007/s00330-020-06801-0 PMID:32193638
13. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study;Luca Carsana, Aurelio Sonzogni, Ahmed Nasr, Roberta Simona Rossi, Alessandro Pellegrinelli, Pietro Zerbi, Roberto Rech, Riccardo Colombo, Spinello Antinori, Mario Corbellino, Massimo Galli, Emanuele Catena, Antonella Tosoni, Andrea Gianatti, Manuela Nebuloni, The Lancet
14. Bai, Tao and Tu, Shengjin and Wei, Yuan and Xiao, Li and Jin, Yan and Zhang, Lei and Song, Jun and Liu, Weihua and Zhu, Qingjing and Yang, Ling and Chen, Hua and Hou, Xiaohua, Clinical and Laboratory Factors Predicting the Prognosis of Patients with COVID-19: An Analysis of 127 Patients in Wuhan, China (2/26/2020). – Google Search [Internet].
15. Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020 Jul;84:106504. https://doi.org/10.1016/j.intimp.2020.106504 PMID:32304994
16. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al.; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries [- Google Search]. JAMA. 2016 Feb;315(8):788–800. https://doi.org/10.1001/jama.2016.0291 PMID:26903337
17. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;
18. Gibson et al. – 2020 – COVIDspan.pdf [Internet].
19. Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, et al. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic: A Multinational Consensus Statement From the Fleischner Society. Chest. 2020 Jul;158(1):106–16. https://doi.org/10.1016/j.chest.2020.04.003 PMID:32275978
20. Sun Z, Zhang N, Li Y, Xu X. A systematic review of chest imaging findings in COVID-19. Quant Imaging Med Surg. 2020 May;10(5):1058–79. https://doi.org/10.21037/qims-20-564 PMID:32489929
21. Litmanovich DE, Chung M. R Kirkbride R, Kicska G, P Kanne J. Review of Chest Radiograph Findings of COVID-19 Pneumonia and Suggested Reporting Language. J Thorac Imaging. 2020 Jun. https://doi.org/10.1097/RTI.0000000000000541.
22. Spiro JE, Sisovic S, Ockert B, Böcker W, Siebenbürger G. Secondary tension pneumothorax in a COVID-19 pneumonia patient: a case report. Infection. 2020 Dec;48(6):941–4. https://doi.org/10.1007/s15010-020-01457-w PMID:32557347
23. Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020 Jun;295(3):715–21. https://doi.org/10.1148/radiol.2020200370 PMID:32053470
24. Xie P, Ma W, Tang H, Liu D. Severe COVID-19: A Review of Recent Progress With a Look Toward the Future. Front Public Health. 2020 May;8:189. https://doi.org/10.3389/fpubh.2020.00189 PMID:32574292
25. Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020 Jun;382(25):2411–8. https://doi.org/10.1056/NEJMoa2012410 PMID:32379955
26. Medscape. Drugs & Diseases. 01 July 2020.
27. Coronavirus FD. (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. Available at https://www.fda.gov/news. May 1, 2020.
28. FDA. Fact sheet for Healthcare providers emergency use authorization (EUA) of remdesivir (GS-5734TM).fda.gov.
29. Dagens A, Sigfrid L, Cai E, Lipworth S, Cheng V, Harris E, et al. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review. BMJ. 2020 May;369:m1936. https://doi.org/10.1136/bmj.m1936 PMID:32457027
30. Zhao JP, Hu Y, Du RH, Chen ZS, Jin Y, Zhou M, et al. [Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020 Mar;43(3):183–4. PMID:32164084
31. Horby P., Landray M. Low-cost dexamethasone reduces death by up to one third in hospitalized patients with severe respiratory complications of COVID-19 (RECOVERY Trail). Oxford University News Release. June 16, 2020.
32. Michael J. Joyner, Katelyn A. Bruno, Stephen A. Klassen, Katie L. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin Proc. 2020;95.
33. Liu ST, Lin HM, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID-19: A matched control study. medRxiv. 2020 May 22. https://doi.org/10.1101/2020.05.20.20102236.
34. Guideline NIH. [Guideline] NIH. Interleukin-6 Inhibitors. COVID-19 Treatment Guidelines. Available at https://www.covid19treatment guidelines.nih.gov/immune-based-therapy June 11, 2020.
35. Mallinckrodt Supports Investigator-Initiated Study at Massachusetts General Hospital to Assess Effectiveness of Inhaled Nitric Oxide in Patients with Severe Acute Respiratory Distress Syndrome Due to COVID-19. Mallinckrodt plc. Available at http://www.mallinckrodt.com/about.news-and-media/news-detail/?id=26721. 2020 Apr 30
36. National Health Commission (NHC) of the People’s Republic of China. The diagnosis and treatment guide of COVID-19 pneumonia caused by new coronavirus infection 7th Edition, published March 3rd, 2020.
37. Bellerophon Therapeutics announces FDA clears initiation of phase 3 study for iNOpulse inhaled nitric oxide therapy to treat COVID-19. Bellerophon Therapeutics.
38. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar;395(10229):1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3 PMID:32171076
39. Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion [Internet]. Eur Respir J. 2020 May;55(5):2000892. [cited 2020 Jul 9]. https://doi.org/10.1183/13993003.00892-2020 PMID:32299867
40. Whittle et al. Respiratory support for adult patient with COVID-19.JACEP open 2020;1:95-101
41. George L Anesi et al. Coronavirus disease 2019 (COVID-19): Critical care and airway management issues. Uptodate; 2020.
42. Möhlenkamp S, Thiele H. Ventilation of COVID-19 patients in intensive care units. Herz. 2020 Jun;45(4):329–31. https://doi.org/10.1007/s00059-020-04923-1 PMID:32313971
43. Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An Index Combining Respiratory Rate and Oxygenation to Predict Outcome of Nasal High-Flow Therapy. Am J Respir Crit Care Med. 2019 Jun;199(11):1368–76. https://doi.org/10.1164/rccm.201803-0589OC PMID:30576221
44. Mechanical ventilation protocol for COVID-19; Saudi MOH. Modified 13May 2020.
45. Mengqiang Luo, M.D.; Shumei Cao, M.D.; Liqun Wei, M.D.; Rundong Tang, M.D.; Shu Hong; et al. Precautions for Intubating Patients with COVID-19; Anesthesiology 6 2020, Vol.132, 1616-1618.
46. Meng L, Qiu H, Wan L, Ai Y, Xue Z, Guo Q, et al. Intubation and Ventilation amid the COVID-19 Outbreak: wuhan’s Experience. Anesthesiology. 2020 Jun;132(6):1317–32. https://doi.org/10.1097/ALN.0000000000003296 PMID:32195705
47. Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 Jun;46(6):1099–102. https://doi.org/10.1007/s00134-020-06033-2 PMID:32291463
48. Gattinoni L, Giosa L, Bonifazi M, Pasticci I, Busana M, Macri M, et al. Targeting transpulmonary pressure to prevent ventilator-induced lung injury. Expert Rev Respir Med. 2019 Aug;13(8):737–46. https://doi.org/10.1080/17476348.2019.1638767 PMID:31274034
49. Walling PT, Savege TM. A comparison of oesophageal and central venous pressures in the measurement of transpulmonary pressure change. Br J Anaesth. 1976 May;48(5):475–9. https://doi.org/10.1093/bja/48.5.475 PMID:1276021
50. Interim guidance document WHO: Clinical management of severe acute respiratory infection when novel corona virus is suspected: what to do or what not to do. Dt 7/4/20.
51. Dondorp AM, Hayat M, Aryal D, Beane A, Schultz MJ. Respiratory Support in COVID-19 Patients, with a Focus on Resource-Limited Settings. Am J Trop Med Hyg. 2020 Jun;102(6):1191–7. https://doi.org/10.4269/ajtmh.20-0283 PMID:32319424
52. Hanidziar D, Bittner EA. Sedation of Mechanically Ventilated COVID-19 Patients: Challenges and Special Considerations. Anesth Analg. 2020 Jul;131(1):e40–1. https://doi.org/10.1213/ANE.0000000000004887 PMID:32392023
53. Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al.; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013 Jun;368(23):2159–68. https://doi.org/10.1056/NEJMoa1214103 PMID:23688302
54. Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit Care Med. 2020 Jun;48(6):e440–69. https://doi.org/10.1097/CCM.0000000000004363 PMID:32224769
55. MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020 Apr;323(13):1245–6. https://doi.org/10.1001/jama.2020.2342 PMID:32074258
56. Yang X, Yu Y, Xu J et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir 2020. Med. https://doi.org/10.1016/S2213-2600(20)30079-5.
57. Klompas M. What is new in the prevention of nosocomial pneumonia in the ICU? Curr Opin Crit Care. 2017 Oct;23(5):378–84. https://doi.org/10.1097/MCC.0000000000000443 PMID:28759469
58. Beach SR, Praschan NC, Hogan C, Dotson S, Merideth F, Kontos N, et al. Delirium in COVID-19: A case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry. 2020 Jul – Aug;65:47–53. https://doi.org/10.1016/j.genhosppsych.2020.05.008 PMID:32470824
59. Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–70. https://doi.org/10.1056/NEJMc2008597.
60. Desforges M, Le Coupanec A, Brison É, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. InInfectious Diseases and Nanomedicine I. New Delhi: Springer; 2014. pp. 75–96.
61. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 Jul;87:18–22. https://doi.org/10.1016/j.bbi.2020.03.031 PMID:32240762
62. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb;395(10223):507–13. https://doi.org/10.1016/S0140-6736(20)30211-7 PMID:32007143
63. Machado C, Gutierrez JV. Brainstem Dysfunction in SARS-COV2 Infection Can Be a Potential Cause of Respiratory Distress.
64. Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020 May;5(1):84. https://doi.org/10.1038/s41392-020-0191-1 PMID:32467561
65. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004 Apr;291(14):1753–62. https://doi.org/10.1001/jama.291.14.1753 PMID:15082703
66. Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018 Nov;33(11):1428–57. https://doi.org/10.1002/gps.4823 PMID:29278283
67. Mascolo A, Berrino PM, Gareri P, Castagna A, Capuano A, Manzo C, et al. Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: a review article. Inflammopharmacology. 2018 Oct;26(5):1141–9. https://doi.org/10.1007/s10787-018-0498-5 PMID:29948492
68. LaHue SC, James TC, Newman JC, Esmaili AM, Ormseth CH, Ely EW. Collaborative delirium prevention in the age of COVID-19. J Am Geriatr Soc. 2020 May;68(5):947–9. https://doi.org/10.1111/jgs.16480 PMID:32277467
69. Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001 Jul;29(7):1370–9. https://doi.org/10.1097/00003246-200107000-00012 PMID:11445689
70. van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ. Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med. 2009 Jun;37(6):1881–5. https://doi.org/10.1097/CCM.0b013e3181a00118 PMID:19384206
71. Maldonado JR, Sher YI, Benitez-Lopez MA, Savant V, Garcia R, Ament A, et al. A study of the psychometric properties of the “Stanford proxy test for delirium” (S-PTD): a new screening tool for the detection of delirium. Psychosomatics. 2020 Mar – Apr;61(2):116–26. https://doi.org/10.1016/j.psym.2019.11.009 PMID:31926650
72. Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020 Jun;250:117583. https://doi.org/10.1016/j.lfs.2020.117583 PMID:32217117
73. Kawada K, Ohta T, Tanaka K, Miyamura M, Tanaka S. Addition of suvorexant to ramelteon therapy for improved sleep quality with reduced delirium risk in acute stroke patients. J Stroke Cerebrovasc Dis. 2019 Jan;28(1):142–8. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.09.024 PMID:30322756
74. Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID-19 (Coronavirus disease 2019) treatment. Circulation. 2020. https://doi.org/10.1161/CIRCULATIONAHA.120.047521.
75. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020 Jul;583(7816):459–68. https://doi.org/10.1038/s41586-020-2286-9 PMID:32353859
76. Schetz JA, Perez E, Liu R, Chen S, Lee I, Simpkins JW. A prototypical Sigma-1 receptor antagonist protects against brain ischemia. Brain Res. 2007 Nov;1181:1–9. https://doi.org/10.1016/j.brainres.2007.08.068 PMID:17919467
77. Sher Y, Miller Cramer AC, Ament A, Lolak S, Maldonado JR. Valproic acid for treatment of hyperactive or mixed delirium: rationale and literature review. Psychosomatics. 2015 Nov-Dec;56(6):615–25. https://doi.org/10.1016/j.psym.2015.09.008 PMID:26674479

 ORCID
ORCID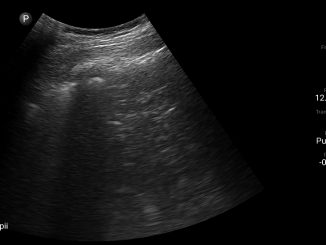

 Creative Commons Attribution
Creative Commons Attribution