Edmundo Gónima Valero1, Federico Vargas2, Yulia Daniela Guio2,*, María Andrade2, María Alejandra Zúñiga2, Elen Alvarez2, Lina Paola Reyes2
Recibido: 10-01-2023
Aceptado: 07-03-2023
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 7 pp. 688-696|https://doi.org/10.25237/revchilanestv52n7-10
PDF|ePub|RIS
Manejo del dolor perioperatorio en abdominoplastía
Abstract
Postoperative pain after abdominoplasty can lead to various complications, ranging from atelectasis to deep vein thrombosis, and even chronic pain syndromes. For this reason, it is vital to administer proper analgesic measures to decrease pain, and in turn, avoid pain related postoperative complications. This article aims to describe the available strategies for perioperative pain management in abdominoplasty. This narrative review includes book chapters, case series, observational studies, meta-analyses, and systematic reviews, in Spanish and English, published from 2000 to 2022 in the PubMed, Embase, Proquest and Scopus databases. It was found that abdominoplasty is a frequently performed esthetic procedure that has a variable risk in injuring nerves that neighbor the muscle structures intervened. Likewise, pain during recovery can also increase the incidence of various postoperative complications such as atelectasis. Administering proper analgesic measures in abdominoplasty patients is vital in avoiding many pain related complications.
Resumen
Antecedentes: El dolor posoperatorio tras una abdominoplastia puede dar lugar a diversas complicaciones, que van desde la atelectasia a la trombosis venosa profunda, e incluso a síndromes de dolor crónico. Por este motivo, es vital administrar medidas analgésicas adecuadas para disminuir el dolor y a su vez, evitar las complicaciones posoperatorias relacionadas con este. Métodos: Revisión narrativa acerca del manejo de dolor perioperatorio en abdominoplastia, incluyendo la anatomía y fisiología relevante, y recomendaciones perioperatorios usando artículos en español y ingles desde 2000 a 2022 en los bases de datos Pubmed, Embase, Proquest y Scopus usando las palabras mesh y decs “abdominoplastia”, “anatomía ”, “analgesia”, “anestesia”, “periodo perioperatorio” y “dolor”, “complicaciones” usando capítulos de libros, series de casos, estudios observacionales, metaanálisis y revisiones sistemáticas. Resultados: La abdominoplastia es un procedimiento estético frecuentemente realizado, en el que se pueden lesionar nervios vecinos a las estructuras musculares intervenidas. Asimismo, el dolor durante la recuperación también puede aumentar la incidencia de diversas complicaciones posoperatorias como la atelectasia. Actualmente, existen muchas estrategias para proporcionar una analgesia adecuada a estos pacientes, como la analgesia multimodal con diversos analgésicos con o sin opioides, técnicas de anestesia regional, bombas de infusión de analgésicos, entre otras. Conclusiones: La administración de medidas analgésicas adecuadas en pacientes sometidos a abdominoplastia es vital para evitar muchas complicaciones relacionadas con el dolor. En cuanto a la medicación, se debe considerar una estrategia multimodal, intentando utilizar la menor cantidad posible de opioides e incluyendo bombas de infusión de analgésicos. Asimismo, las estrategias regionales como el bloqueo de los erectores espinales, del plano transverso del abdomen y del cuadrado lumbar son importantes para disminuir adecuadamente el dolor posoperatorio.
-
Introduction
Abdominoplasty is one of the most performed procedures by plastic surgeons around the world, with a significant case increase due to the number of obese patients currently undergoing bariatric surgery, therefore it is important to understand its complications, as well as pain management in this type of surgery[1]. It has been described that the rate of complications for this procedure ranges between 0.43% – 4.7%[2]. Although there are minor complications, these vary between techniques, so it is important to know the main complications, and educate patients in order to offer them realistic expectations of results[3].
Nerve injuries are one of the most frequent complications, due to the dissection that is performed when raising the abdominal flap[4]. Another complication associated with abdominoplasty is deep vein thrombosis (DVT), which, despite having a low incidence, is the esthetic procedure that is most likely to develop it. DVT can be caused by excessive plication of the rectus abdominis, poorly controlled pain that leads to poor mobility of the patient, and indiscriminate administration of fluids, among others[5],[6].
Furthermore, it is important to remember that pain generates a cascade and release of inflammatory mediators such as prostaglandins and leukotrienes, as well as an endocrine and metabolic response. This leads to a release of hormones that produces harmful effects on the different tissues and, in turn, generates an increase in patient morbidity and mortality and slows down recovery. There are also important implications that this may have on the patient’s mental health[7].
As mentioned above, inadequate perioperative and postoperative pain management, in addition to exacerbating certain complications derived from abdominoplasty, can also be a risk factor for the development of DVT. For this reason, it is vital to understand the different therapeutic strategies that are currently available, their indications and complications, to comprehensively address these patients whose expectations can be ruined by a subsequent complication or poor pain management.
Therefore, the objective of this article is to describe the current literature on analgesic techniques for the adequate management of perioperative pain in abdominoplasty, considering that in recent years innovative options have emerged, and with their combined use can be effective in controlling pain in this context.
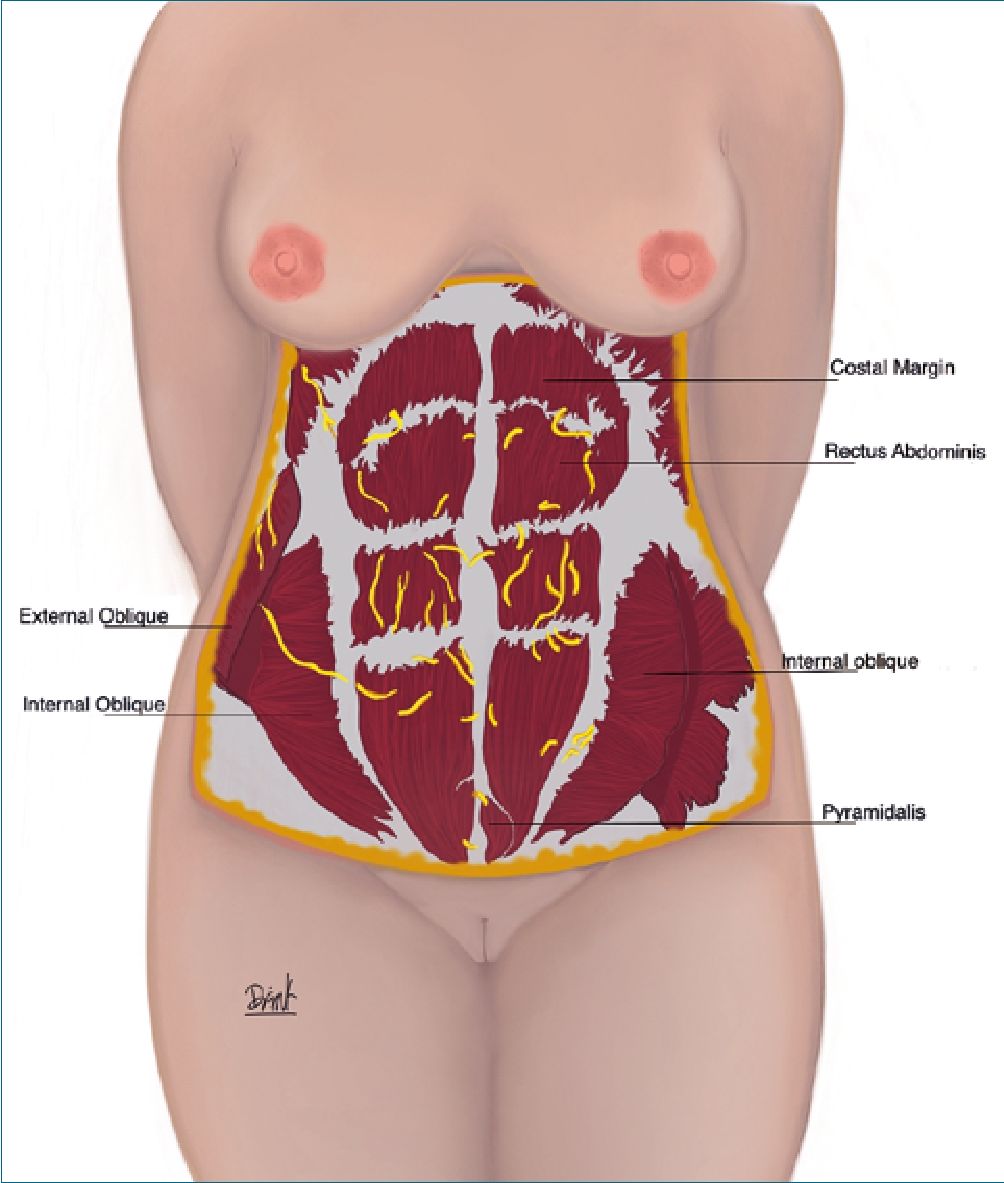
Figure 1. Anterolateral muscles of the abdomen. Knowledge of the abdominal wall anatomy is important for proper flap dissection during abdominoplasty.
-
Methods
Narrative review on the management of perioperative pain in abdominoplasty, including the relevant anatomy and physiology, and perioperative recommendations using articles in Spanish and English from 2000 to 2022 in the PubMed, Embase, Proquest and Scopus databases using the keywords “Pain Management”, “analgesia”, “abdominoplasty”, “Plastic Surgery”, “anatomy”, “anesthesia”, “perioperative period” and “complications”. Using book chapters, case series, observational studies, meta-analyses, and systematic reviews.
-
Main body
-
The Abdominal Wall
The results of abdominoplasty are related to adequate knowledge of anatomy and surgical training. Skin resection must be preceded by a plan that allows the blood supply of the abdominal flap to be preserved. The most relevant structures of the abdominal wall involved in an abdominoplasty include the muscular and fascial anatomy, the adhesion zones, the nerves and blood supply to the flap, fat, and the navel[2],[8].
The abdominal wall is made up of five muscles:
– Rectus abdominis and pyramidalis muscle which occupy the anteromedial part of the abdomen.
– Internal, external and transverse oblique muscle of the abdomen; located from the posterolateral to the anterior wall of the abdomen, forming the rectus sheath (Figure 1 and 2).
-
Nerves
The innervation of the abdominal wall is given by the cutaneous branches of the intercostal nerves T7-T12. Additionally, other nerves that can be dissected during abdominoplasty are the ilioinguinal and iliohypogastric, which are in the lateral portion of the abdomen and could generate sensory problems at the level of the thigh and groin (Figure 2).
-
Blood supply
As for the abdominal wall blood supply, it is irrigated by arteries of the thorax and the pelvic region, these two important networks anastomose and are important for the supply of the flap during dissection. Knowledge of the irrigation of the abdominal wall makes it possible to avoid ischemic complications secondary to the procedure[8](Figure 3).
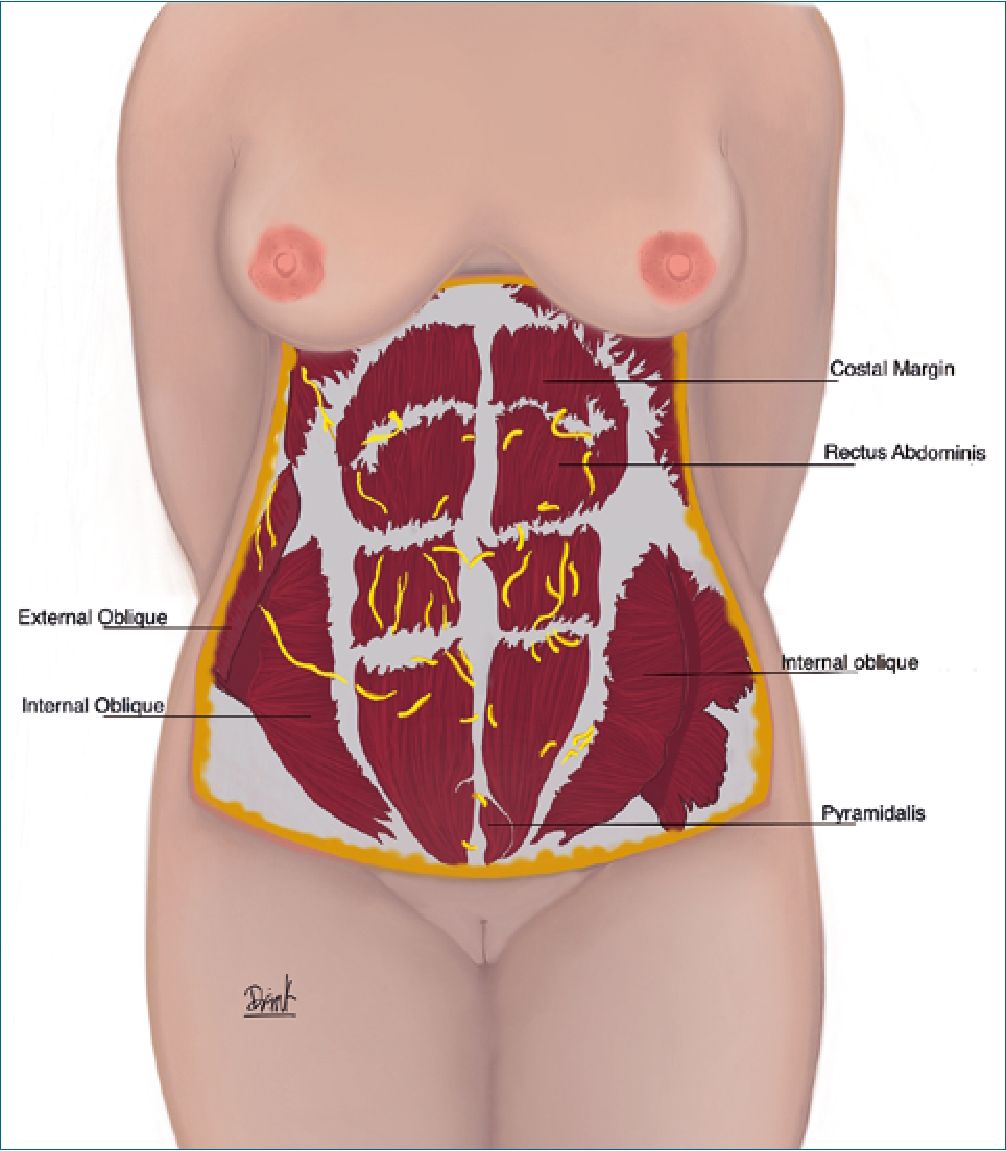
Figure 2. Nerves of the abdominal wall. The innervation of the abdominal wall is given by the cutaneous branches of the intercostal nerves T7- T12. The nerves that are most frequently dissected during an abdominoplasty are lateral femoral cutaneous, ilioinguinal and iliohypogastric.
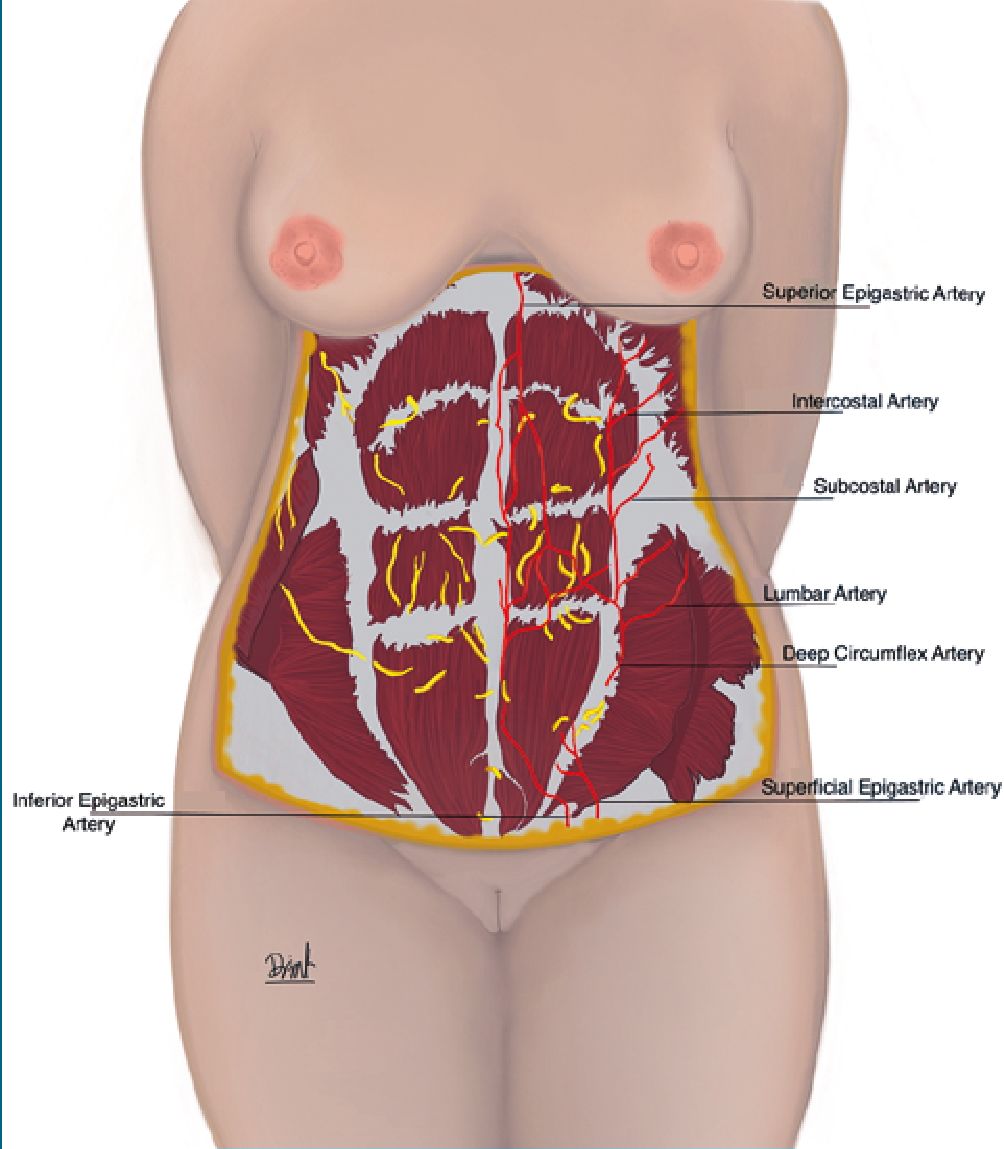
Figure 3. Irrigation of the abdominal wall. The abdominal wall is irrigated by arteries of the thorax and the pelvic region.
-
Abdominoplasty and its implications on thoraco-abdominal anatomy and pain
Abdominoplasty is one of the most frequently requested surgical procedures in plastic surgery, as it improves the esthetic appearance of the abdomen, removing excess skin and improving the quality of the abdominal muscles. In the United States, in 2020 it was the ninth most performed procedure[1].
Likewise, it is likely that the demand for this procedure will continue to increase due to the large number of obese patients who today undergo bariatric surgery[9], which is why it is important to know and identify the possible complications that may arise from this surgical intervention and negatively affect the result or delay the recovery of the patient[9].
Multiple authors have described certain events derived from this procedure. Swason et al., describe that the rate of complications for abdominoplasty as 4.7%, while other researchers report a rate of 0.43%[2].
Complications can be classified into:
• Local [seroma, hematoma, surgical site infection, necrosis, hypertrophic or keloid scars].
• Systemic, such as venous thromboembolism.
• According to its time of appearance.
*Immediate such as deep venous thrombosis and pulmonary embolism, which are infrequent, but compromise the patient’s life.
*Early [seen after the first postoperative month has elapsed]; such as hematoma, seroma, infection, necrosis or wound dehiscence.
*Late such as hypertrophic scar, diastasis and chronic neu- ropathy[1],[9].
Although some complications may be more serious and bothersome than others, they all negatively affect the postoperative quality of life of patients[10]. However, in this article we will focus on the complications related to inappropriate pain management and an inadequate surgical technique that can cause greater discomfort to the patient.
-
Nerve Lesions
The loss or diminution of cutaneous sensitivity in the abdominal region is a consequence of the lesion of sensory nerves coming from the anterior and lateral branches of the costal nerves and the branches of the iliohypogastric and ilioinguinal nerves (Figure 2), due to the dissection that is performed to elevate the abdominal flap. 11 The risk of causing nerve damage is approximately 1.94%[4].
The mechanisms by which nerve damage occurs are direct (caused by the suture or scalpel) and indirect (secondary to entrapment of the nerve during the healing process).4,9 Depending on the mechanism of the injury, the pathophysiology varies; in the case of transection, it can cause a stump neuroma. Continuity neuroma can occur in the case of a mixed or incomplete nerve injury, and compression neuropathy is secondary to the normal healing process or tissue edema[4].
-
Femoral cutaneous nerve
The nerves that are most frequently injured during an abdominoplasty are: the lateral femoral cutaneous nerve in 1.36% of the cases reported by Ducic et al.[4].Of these, only 25% had the symptoms resolved, the remaining percentage presented a permanent injury to this nerve. Chopra et al., described that the lateral femoral cutaneous nerve was injured in 9.8% of cases, so it is pertinent to be extremely careful during the extension of the inferior lateral incision, which is where the nerve passes medial to the anterior superior iliac spine[12].
-
Iliohypogastric nerve
The iliohypogastric nerve is the second most injured, with a risk of 0.10%. It is recommended to carry out an exhaustive pre-surgical planning, considering that the risk of causing nerve injuries increases with the prolongation of surgery and multiple procedures[4].
Risk factors that may be related to chronic pain in these patients are a history of bariatric surgery, poor management of acute postoperative pain, and major complications derived from the procedure[13]Severo et al., carried out a study in which they evaluated fifty patients who underwent abdominoplasty and found that all patients presented some alteration in sensitivity, mainly in the hypogastrium. Three and six months after the procedure, there was an absence of light touch, pain, and two-point discrimination. The mesogastrium was the second most affected area and thermal sensitivity was irreversibly compromised[5].
Farah et al., found the same results as in the previously mentioned study, where sensitivity was affected in all its modalities, mainly in the hypogastrium, and there was a decrease in temperature sensitivity in the suprapubic area or incision site[14].
Likewise, Presman and collaborators conducted a study in which, through a questionnaire applied to 217 people who had undergone abdominoplasty, issues related to pain and sensitivity in the abdominal skin were evaluated. It was found that 8.2% of the patients persisted with pain between three months and 9 years after the procedure. This complication was strongly associated with dissatisfaction with surgery. Additionally, the authors intuited that neuropathic pain was the main reason for the patient’s sensory disturbance. However, despite this hypothesis, none of the patients were treated with medications recommended for the relief of neuropathic pain (tricyclic antidepressants, pregabalin, gabapentin). Finally, they propose early pain management as a method to reduce the risk of developing chronic neuropathic pain[9].
Although it is true that anatomical knowledge and adequate surgical skill can reduce the risk of direct nerve injury, authors such as Ducic et al., suggest that there are injuries that are difficult to avoid. This can be a result of unusual anatomical variations or injuries caused by nerve entrapment secondary to the healing process[4].
-
Pulmonary Function
Literature suggests that pulmonary complications could be derived from excessive plication of the rectus abdominis that is associated with chronic postoperative pain. The plication generates an increase in intra-abdominal pressure (IAP) (pathological increase > 12 mmHg or more), so there is a decrease in venous return and stasis, which predisposes to developing deep vein thrombosis. On the other hand, it can cause an increase in intrathoracic pressure, generating a poor ventilatory pattern[6],[15],[16].
In a prospective study carried out by Pereira et al., where they measured IAP and pulmonary compliance in patients who underwent abdominoplasty, they observed that, in effect, plication of the rectus abdominis generates an increase in IAP, affecting lung compliance[6]. Other factors related to the decrease in lung volumes are pain, the patient’s fear of injury, the administration of anesthetics, visceral manipulation and the indiscriminate administration of intravenous fluids, altering the respiratory dynamics (congestion lung, reduced volumes and oxygenation)[17].
-
Venous thromboembolism
The incidence of thromboembolism after abdominoplasty is 0.02 to 1.5% according to various authors[1],[16],[18]. Although the incidence is low, abdominoplasty is the esthetic procedure that is most likely to develop venous thromboem- bolism[18]. The risk is increased when patients have a body mass index greater than 30 kg/m2. It is important to assess the thromboembolic risk of each patient and determine the need for thromboprophylaxis or preventive measures. It is recommended to use the Caprini scale to stratify risk and only administer thromboprophylaxis in patients with a score greater than eight[18].
Regarding preventive measures, compression stockings are described from the intraoperative period until the patient is discharged, intermittent pneumatic compression devices and early assisted ambulation. For the latter, it is vital to implement adequate pain management that allows deambulation without generating major complications and discomfort to the pa- tient[1],[16]. Likewise, the proper pain management will allow the patient to not feel fear from movement and allow more ease of walking and movement after surgery.
-
Implications of post-surgical pain
Pain perception involves both the central (spinal and supraspinal) and peripheral nervous systems. This is classified into different types: Nociceptive (visceral and somatic), neuropathic, mixed and nociplastic[19]. Somatic pain is the most common in abdominoplasty due to direct damage to the tissue, along with neuropathic pain due to direct damage to the nerve.
Postoperative pain is considered acute pain, which is the result of inflammation and tissue trauma caused by the surgical procedure (incision, dissection, burns, direct nerve injury) [20]. This generates an endocrine and metabolic response, as well as the release of inflammatory mediators (prostaglandins, leukotrienes, histamine, bradykinins, interleukins IL-2 and IL-6), which in turn increase the levels of hormones such as cortisol, aldosterone, glucagon, among others[20].
This response stimulates the somatic pathway, increasing the release of hormones from the hypothalamus and in turn stimulating secretion from the anterior and posterior pituitary. Autonomic hyperactivity results in increased heart rate, peripheral vascular resistance, blood pressure, and myocardial contractility, leading to increased cardiac output and oxygen consumption, with increased risk of myocardial ischemia and infarction. By increasing the release of antidiuretic hormone, water and salt retention occurs in the renin-angiotensin system, which contributes to this increased load on the cardiovascular system[7],[21].
As for pulmonary changes, they are produced by an involuntary spinal reflex response to the noxious stimulus of the injured area, generating a reflex muscle spasm of the respiratory and abdominal muscles. This leads to a restriction of chest mobility, decreasing tidal volume and inspiratory reserve capacity, causing hypoventilation, hypoxia, increased bronchial secretions, and risk of atelectasis. Additionally, this predisposes to tracheobronchial and pulmonary infections. In addition, diaphragmatic dysfunction may occur, which further impairs ventilation, decreasing total vital capacity by 40%-60%[22].
Increased sympathetic activity increases gastrointestinal secretions and smooth muscle sphincter tone, decreasing intestinal motility, leading to gastric stasis and paralytic ileus; this adrenergic response can cause mucosal ischemia. In addition to this, opioid analgesics contribute to the decrease in intestinal motility[22].
In the genitourinary tract, there is an increase in the tone of the urinary sphincter, as well as a reflex inhibition of the tone of the bladder with subsequent urinary retention, which favors infections. This increased sympathetic activity stimulates the area postrema, increasing both nausea and emetic episodes[22].
At the metabolic level, a state of hypercoagulability, venous stasis, an increased risk of deep vein thrombosis and pulmonary thromboembolism will be found, which is further aggravated by reduced physical activity. Increased cortisol can also cause hyperglycemia. In the immune system there is a deterioration of function, since pain suppresses both cellular and humoral function and causes lymphopenia, leukocytosis and depression of the reticuloendothelial system. Cortisol, as a mediator of the stress response, as well as other mediators, are potent immunosuppressants, which decreases resistance to pathogens and can generate an increased risk of infection. In the musculoskeletal system, due to inactivity, weakness, limitation of movement, muscle atrophy and fatigue are generated[23].
This response to pain at the bodily level is not only limited in physical aspects but also has mental implications such as increased anxiety, fear and anger. Finally, all these effects generated by pain cause delayed recovery, delay in the normalization of daily life, and greater use of resources and costs of medical care[7]. With all of this in mind, it is vital to understand the proper analgesic management in these patients to avoid any pain-related complications.
-
Anesthetic management in abdominoplasty
Although general anesthesia is the technique of choice for this type of procedure and is usually managed with remifentanil and sevoflurane or desflurane, neuraxial techniques have also been described (subarachnoid and epidural). They have
the benefits of less pain in the immediate postoperative period, suppression of the response to surgical stress, preservation of perioperative immune function, oxygenation, pulmonary functional residual capacity, improvement of visceral blood flow and less incidence of venous thromboembolic disease[24]. Another technique described in the literature is TIVA (total intravenous anesthesia), which has a lower incidence of postoperative nausea and vomiting, faster recovery, and less agitation on awakening.
-
Pharmacological management
Multimodal analgesia consists of the administration of different drugs with various mechanisms of action (NSAIDs, paracetamol, dipyrone, opioids, among others), to obtain maximum efficacy, achieve postoperative pain control and reduce adverse effects. The pain threshold of each patient must be taken into account, avoiding central and peripheral sensitization, such as the expansion of the nociceptive message produced by the surgeries performed[25]. A combination of various locore- gional techniques based on local anesthetics with or without the addition of short-acting opioids can be used[26].
-
Management with opioids
Despite the advances that can be evidenced in pain management, the fundamental pillar continues to be the use of opioids, since these bind to receptors in the central nervous system and peripheral tissues, generating a modulation of the effect of nociceptors[27]. However, they are associated with opioid-induced respiratory depression (OIRD) and gastrointestinal complications, which is why it must be managed at an adequate dose that does not impede the benefit for the patient[28].
They can be administered orally, transdermally, parenterally, neuraxially, and rectally. The most commonly used intravenous opioids for postoperative pain are morphine, hydromorphone, and fentanyl. Morphine is the standard option and is widely used. Fentanyl and hydromorphone are synthetic derivatives of morphine and are more potent, have a shorter onset of action, and shorter half-lives compared to morphine[27].
-
Management without Opioids
There are several coadjuvant medications in the management of postoperative pain, generating a lower consumption of opioids. Ketorolac is an injectable nonsteroidal anti-inflammatory drug with analgesic properties that acts on COX-1 and is generally used for preventive analgesia and as an adjunct to other agents. It reduces the consumption of opioids between 25 and 45%[29].
Paracetamol is a centrally acting analgesic that has been shown to be a weak inhibitor of COX1, COX2 and COX3, being slightly more sensitive to COX 2, additionally it has effects on serotonergic mechanisms that produce pain inhibition[25]. It has also been known that paracetamol can block prostaglandin E2 synthase by reducing factors such as glutathione, blocking the action of COX peroxidases and interacting with serotonergic inhibitory pathways. Systematic reviews of randomized controlled trials (RCTs) confirm the efficacy of oral paracetamol for acute pain. However, it has a slow onset of analgesia but has been associated with reduced postoperative pain and opioid use[29].
-
Local anesthetics
It is important to highlight this pharmacological group, since local anesthetics will play a relevant role in regional anesthesia. With its ability to reversibly block nerve impulse conduction, it leads to a loss of sensitivity, without removing nerve function, which tends to recover completely after the effect[30].
As part of multimodal analgesia, infiltrations with local anesthetic can be implemented prior to the surgical procedure, in order to potentiate the analgesic effect and provide an opioidsparing approach[31],[32].
Bupivacaine is one of the most widely used local anesthetics, as it is more powerful and long-lasting than others such as lidocaine[33]. Liposomal bupivacaine uses lipid chambers that encapsulate the drug and prolong analgesia. However, the described advantage of “slow release” can also be its disadvantage; Unlike bupivacaine in saline, liposomal bupivacaine does not diffuse well into tissues, which probably explains its inconsistent effectiveness. In contrast, administration of bupivacaine in saline allows the anesthetic to easily penetrate tissue planes, including the rectus sheath, making subfascial injections unnecessary[33].
It has been shown that choosing analgesia by local infiltration with general anesthesia in patients undergoing abdominoplasty has reduced postoperative complications, as well as pain and adverse effects of the analgesics administered, since lower doses are used than those necessary when compared in a setting where local anesthetics are not used[32],[34].
Another use with local anesthetics is the combination of diluted local anesthesia used along the incision as well as large volumes injected into the fascia, allowing the procedure to be performed under conscious sedation. Evidence was found in the study by Kryger et al., evaluating injections of 0.5% lidocaine with epinephrine into nerves as they pierce the rectus fascia. As a result, greater patient satisfaction was obtained after this technique, with a high probability that they would undergo cosmetic procedures with assisted local anesthesia again[35]. There is also literature that exposes adequate analgesia for procedures such as mini abdominoplasty, achieving coverage for at least 4 hours after surgery through local tissue infiltration with ropivacaine or levobupivacaine; with optimal results in relation to the intensity and duration of analgesia when the anesthetic of choice was levobupivacaine[36].
-
Infusion pumps for pain management
As has been mentioned, an additional alternative is pain pumps, which are responsible for providing patient-controlled analgesia (PCA)[33].
Additionally, the efficacy of perineural catheters with a PCA pump has been studied in patients with massive weight loss undergoing a body contouring abdominoplasty, finding that the use of local anesthesia catheters for pain in abdominoplasty may be associated with a decreased use of opioids and could result in a shorter hospital stay[32].
Unlike injection or local anesthetic blocks, there is also evidence in favor of the use of infusion devices that deliver local anesthetic directly to the operative site, as they have improved postoperative analgesia in various plastic surgery procedures, including abdominoplasty. To ensure an optimal analgesic effect, it is important to follow the recommendations regarding the technique and placement of the infusion catheters. In the
case of abdominoplasty, this is usually accomplished by suturing the catheters to the rectus sheath or by another sutureless method by drawing the catheter to a subfascial plane, delivering anesthesia directly to the rectus muscles; achieving excellent postoperative analgesia with securely attached catheters while the abdominoplasty wound is being closed, as well as during the immediate postoperative period[37].
In a randomized controlled trial, 20 women underwent abdominoplasty with plication of the rectus abdominis. They were randomly divided into two groups, an experimental group that received analgesic management with a disposable pump for incisional pain management and a control group. The pump provided a continuous supply of 0.5% bupivacaine placed directly in the abdominal fascia. It was shown that after the operation, the patients with the pain pump were able to walk 21.6 hours after surgery compared to 40.8 hours in the control group; Additionally, it was highlighted that patients in the experimental group consumed fewer postoperative opioids[38]. Similarly, in another study, postoperative use of pain pumps in abdominoplasty patients significantly improved pain modulation, with pain pump patients experiencing a statistically significant decrease in perceived pain compared to those without pain pumps. Still, postoperative pain control after cosmetic plastic surgery has been achieved by systemic administration of various opioid analgesics[28].
However, in a retrospective study they evaluated pain management in 73 patients who had undergone abdominoplasty, 38 patients were managed with local anesthetic pumps for pain and 35 without a pump; found that in the pain pump group, there was a small but not statistically significant reduction in pain medication use (2.65 versus 3.04 pain units) (p = 0.34). Interestingly, pain scores were higher in the pain pump group, but not statistically significant (2.73 vs 2.31) (p = 0.17)[39].
From this it can be noted that the use of PCA pumps for pain management after abdominoplasty is an effective method to significantly reduce both the amount of pain experienced by patients and the amount of opioids used postoperatively[3],[4].
-
Regional anesthesia
-
Epidural and spinal anesthesia
In a prospective randomized study, 200 patients undergoing abdominoplasty were evaluated. Where 100 patients were operated under general anesthesia and another 100 patients were operated under spinal anesthesia. Spinal anesthesia was shown to be an effective anesthetic technique for abdominoplasty, with fewer postoperative complications compared to general anesthesia[24].
Epidural efficacy in belt lipectomies was analyzed in a study with 62 patients who underwent this procedure, showing that mean and maximum pain scores, as well as the use of opiates, were significantly lower in the experimental group. Before surgery, an epidural was placed in the lower thoracic region and an intraoperative infusion consisting of a combination of local analgesia and opiate was started. On postoperative days 0 and 1, the mean pain score was 1.53 and 1.84 on the visual analog scale versus 3.64 and 3.60 in the control group, respectively. Mean non-epidural opioids in morphine equivalents were 2.0 mg and 18.0 mg on postoperative days 0 and 1, respectively, in the epidural cohort versus 17.0 mg and 27.3 mg in the control group[40].
-
Transverse abdominis plane block
Furthermore, it was evidenced in this study that ultrasound- guided transverse abdominal plane (TAP) blocks provide effective analgesia after lipoabdominoplasty, allowing for more convenient early postoperative mobilization and decreasing need for opioids, as well as their related side effects. Therefore, it is suggested that ultrasound-guided TAP block be considered in most lipoabdominoplasty cases to improve the patient experi- ence[41].
In a study conducted at Ain Shams University Hospitals, the result was that ultrasound-guided TAP block allows direct visualization of all the anatomical structures via ultrasound, which increases the safety margin, optimizes the quality of the block, and reduces the need for systemic analgesia[42].
It can be noted that the TAP block appears to be very promising for patients undergoing abdominoplasty, as it provides effective postoperative analgesia in the first 12 hours postoperatively of a major abdominoplasty. In this study, all patients in the TAP block group reported lower levels of pain with their postoperative analgesic regimen, which was demonstrated in their recovery rates.4 A prospective randomized study was analyzed to explore the hypothesis that the addition of ketamine (0.5 mg/kg) to levobupivacaine 0.5% in ultrasound-guided TAP block would lead to better and longer duration of postoperative analgesia in patients undergoing abdominoplasty (Experimental: Levobupivacaine 0.5% 15 ml plus ketamine 0.5 mg/kg in a total volume of 20 ml VS Control: levobupivacaine 0.5% 15 ml plus 5 ml normal saline in a total volume of 20 ml). It was concluded that in TAP blockade, the addition of ketamine improved the analgesic efficacy of levobupivacaine in patients undergoing abdominoplasty and reduced the necessary analgesics in the postoperative period[43].
-
Erector spinae block
Ultrasound-guided erector spinae block (ESP) is considered an excellent analgesic technique. In a prospective randomized study, two groups of patients were compared in which 25 patients received ESP blockade and the other group 26 received TAP blockade. It was shown that compared to the TAP block, the ESP block allows a more reliable pain relief and a longer analgesic period[44].
-
Quadratus lumborum block
The quadratus lumborum block (QLB) has been seen in use recently within this population but has shown mixed results. A study done by Bjelland et al. compared patients undergoing abdominoplasty who received QLB bilaterally to those who did not, and found that the blockade did not provide significant benefit when looking at reduction of opioid requirements[46]. Conversely, a later study done by Meocuhy el al. conducted a similar study in abdominoplasty patients, and found that the QLB reduced postoperative pain and opioid consumption, leading to improved quality of recovery[47]. Therefore, further studies are required in order to arrive at a concrete solution regarding the use of the QLB in the population[47].
-
Paravertebral block
The paravertebral block in abdominoplasty may prove to be useful, however there are various significant side effects such as epidural or spinal spread of the local anesthetic, as well as a
risk for pneumothorax, which may deter anesthesiologists from performing this block in an ambulatory setting[48].
There is a great potential to provide anesthesia and postoperative analgesia with the utilization of paravertebral blocks considering that the usual surgical field is maintained between T6 and T12, which is the usual dermatomal blockage plane for this technique. Currently, there is insufficient literature on the usage of this technique in this surgical population. However, Rudkin et al., describes a case series where the paravertebral block allowed no postoperative opioid necessity, consistently low pain scores, and no nausea or vomiting[49].
-
Conclusions
Administering proper analgesic measures in abdominoplasty patients is vital in avoiding many pain related complications. Concerning medications, a multimodal strategy should be considered with an attempt in using as little opioids as possible, and an inclusion of analgesic infusion pumps. Likewise, regional strategies such as an erector spinae, transverse abdominis plane and quadratus lumborum block are important in adequately decreasing postoperative pain.
-
Author contributions:
Edmundo Gónima, Daniela Guio, María Andrade, María Zúñiga, Elen Quintero, Lina Reyes contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting and editing of the text.
Acknowledgements: We acknowledge Laura Rincon for her assistance with the digital media for this article.
We would like to acknowledge Dr. Sebastian Amaya for his methodological guidance for this review.
Conflict of Interest: The authors declare no conflicts of interest.
Fundig: No funding to declare.
References
1. Vidal P, Berner JE, Will PA. Managing Complications in Abdominoplasty: A Literature Review. Arch Plast Surg. 2017 Sep;44(5):457–68. https://doi.org/10.5999/aps.2017.44.5.457 PMID:28946731
2. Swanson E. Prospective outcome study of 360 patients treated with liposuction, lipoabdominoplasty, and abdominoplasty. Plast Reconstr Surg. 2012 Apr;129(4):965–78. https://doi.org/10.1097/PRS.0b013e318244237f PMID:22183499
3. Nahai FR. Anatomic considerations in abdominoplasty. Clin Plast Surg. 2010 Jul;37(3):407–14. https://doi.org/10.1016/j.cps.2010.03.003 PMID:20624540
4. Ducic I, Zakaria HM, Felder JM 3rd, Arnspiger S. Abdominoplasty-related nerve injuries: systematic review and treatment options. Aesthet Surg J. 2014 Feb;34(2):284–97. https://doi.org/10.1177/1090820X13516341 PMID:24436448
5. Mercedes-Acosta S, Fragoso-Báez A, Sabala R, Matos D, Medina T. Estudio de los trastornos postabdominoplastia de la sensibilidad cutánea superficial. Cirugía Plástica Ibero-Latinoamericana. 2013 Sep;39(3):219–24. https://doi.org/10.4321/S0376-78922013000300002.
6. Pereira N, Sciaraffia C, Danilla S, Parada F, Asfora C, Moral C. Effects of Abdominoplasty on Intra-Abdominal Pressure and Pulmonary Function. Aesthet Surg J. 2016 Jun;36(6):697–702. https://doi.org/10.1093/asj/sjv273 PMID:26895955
7. Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North America. 2005 Mar;23(1):21–36. https://doi.org/10.1016/j.atc.2004.11.013 PMID:15763409
8. da Silveira Carvalho CG, Baroudi R, Keppke EM. Anatomical and technical refinements for abdominoplasty. Aesthetic Plast Surg. 1976 Dec;1(1):217–28. https://doi.org/10.1007/BF01570254 PMID:24173746
9. Presman B, Finnerup K, Andresen SR, Nikolajsen L, Finnerup NB. Persistent Pain and Sensory Abnormalities after Abdominoplasty. Plast Reconstr Surg Glob Open. 2015 Dec;3(11):e561. https://doi.org/10.1097/GOX.0000000000000542 PMID:26893986
10. Lesko RP, Cheah MA, Sarmiento S, Cooney CM, Cooney DS. Postoperative Complications of Panniculectomy and Abdominoplasty: A Retrospective Review. Ann Plast Surg. 2020 Sep;85(3):285–9. https://doi.org/10.1097/SAP.0000000000002220 PMID:32788565
11. van Uchelen JH, Werker PM, Kon M. Complications of abdominoplasty in 86 patients. Plast Reconstr Surg. 2001 Jun;107(7):1869–73. https://doi.org/10.1097/00006534-200106000-00037 PMID:11391211
12. Chopra K, Kokosis G, Slavin B, Williams E, Dellon AL. Painful Complications After Cosmetic Surgery: Management of Peripheral Nerve Injury. Aesthet Surg J. 2019 Nov;39(12):1427–35. https://doi.org/10.1093/asj/sjy284 PMID:30346489
13. Chatel H, Madar Y, Leyder P, Bonneau C, Barrat C, Quilichini J. Prevalence and factors associated with persistent pain following body contouring surgery. J Plast Reconstr Aesthet Surg. 2016 May;69(5):700–5. https://doi.org/10.1016/j.bjps.2016.01.008 PMID:26923660
14. Farah AB, Nahas FX, Ferreira LM, Mendes JA, Juliano Y. Sensibility of the abdomen after abdominoplasty. Plast Reconstr Surg. 2004 Aug;114(2):577–82. https://doi.org/10.1097/01.PRS.0000128356.93462.7B PMID:15277836
15. Fluhr S, Andrade AD, Oliveira EJ, Rocha T, Medeiros AI, Couto A, et al. Lipoabdominoplasty: repercussions for diaphragmatic mobility and lung function in healthy women. J Bras Pneumol. 2019 May;45(3):e20170395. https://doi.org/10.1590/1806-3713/e20170395 PMID:31166554
16. Rangaswamy M. Minimising complications in abdominoplasty: an approach based on the root cause analysis and focused preventive steps. Indian J Plast Surg. 2013 May;46(2):365–76. https://doi.org/10.4103/0970-0358.118615 PMID:24501473
17. Cohen B, Meilik B, Weiss-Meilik A, Tarrab A, Matot I. Intraoperative factors associated with postoperative complications in body contouring surgery. J Surg Res. 2018 Jan;221:24–9. https://doi.org/10.1016/j.jss.2017.08.004 PMID:29229135
18. Keyes GR, Singer R, Iverson RE, Nahai F. Incidence and Predictors of Venous Thromboembolism in Abdominoplasty. Aesthet Surg J. 2018 Feb;38(2):162–73. https://doi.org/10.1093/asj/sjx154 PMID:29117339
19. Cohen Steven P, Raja Srinivasa N. Dolor. In: Lee G, Schafer AI, editors. Goldman-Cecil Tratado de medicina interna [Internet]26th ed. Barcelona: Elsevier; 2021. pp. 128–37. [ [cited 2022 Sep 14]], Available from https://www-clinicalkey-es.ezproxy.unbosque.edu.co/#!/content/book/3-s2.0B9788491137658000278?scrollTo=%23hl0000557
20. Hay D, Nesbitt V. Management of acute pain. Surgery (Oxf). 2019 Aug;37(8):460–6. https://doi.org/10.1016/j.mpsur.2019.05.004.
21. Martinez-Salio A, Zarranz JJ. Dolor. In: Zarranz JJ, editor. Neurología [Internet]6th ed. Barcelona: Elsevier; 2018. pp. 235–48. [ [cited 2022 Sep 14]], Available from https://www-clinicalkey-es.ezproxy.unbosque.edu.co/#!/content/book/3-s2.0-B9788491130710000131?scrollTo=%23hl0000540
22. Brennan TJ. Pathophysiology of postoperative pain. Pain. 2011 Mar;152(SUPPL.3). https://doi.org/10.1016/j.pain.2010.11.005.
23. Asokumar Buvanendran. Timothy R. Lubenow, Jeffrey S. Kroin. Postoperative Pain and Its Management. In: McMahon SB, Koltzenburg M, editors. Wall & Melzack’s Textbook of Pain [Internet]6th ed. Elsevier; 2013. pp. 629–44. [ [cited 2022 Sep 14]], Available from https://www-clinicalkey-es.ezproxy.unbosque.edu.co/#!/content/book/3-s2.0-B9780702040597000462
24. Metry AA, Nakhla GM, Wahba WZ, Wahba RM, Kamel IH. Abdominoplasty under spinal anesthesia: A feasibility study. Anesth Essays Res. 2019;13(2):243–7. https://doi.org/10.4103/aer.AER_69_19 PMID:31198238
25. González de Mejía N. Analgesia Multimodal Postoperatoria. Revista de la Sociedad Española de Dolor. 2005;(Mar):112–8.
26. Tan HS, Habib AS. Oliceridine: A Novel Drug for the Management of Moderate to Severe Acute Pain – A Review of Current Evidence. J Pain Res. 2021 Apr;14:969–79. https://doi.org/10.2147/JPR.S278279 PMID:33889018
27. Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013 Sep;26(3):191–6. https://doi.org/10.1055/s-0033-1351138 PMID:24436674
28. Chavez-Abraham V, Barr JS, Zwiebel PC. The efficacy of a lidocaine-infused pain pump for postoperative analgesia following elective augmentation mammaplasty or abdominoplasty. Aesthetic Plast Surg. 2011 Aug;35(4):463–9. https://doi.org/10.1007/s00266-010-9633-4 PMID:21136251
29. Hemmerling TM. Pain management in abdominal surgery. Langenbecks Arch Surg. 2018 Nov;403(7):791–803. https://doi.org/10.1007/s00423-018-1705-y PMID:30284029
30. Vincent A, Bernard L, Léone M. Farmacología de los anestésicos locales. EMC – Podología. 2019 Oct;21(4):1–19.
31. Šakić K, Bagatin D, Bagatin T, Šakić L, Jeleč V, Včev A. Comparison of Different Surgical Procedures with Local Infiltration Analgesia in Day Surgery. Acta Clin Croat. 2019 Jun;58 Suppl 1:67–73. PMID:31741562
32. Giordano S, Uusalo P, Oranges CM, di Summa PG, Lankinen P. Local anesthetic pain catheters to reduce opioid use in massive weight loss patients undergoing abdominoplasty: A comparative study. J Plast Reconstr Aesthet Surg. 2020 Apr;73(4):770–6. https://doi.org/10.1016/j.bjps.2019.11.003 PMID:31864888
33. Swanson E. A Physiologic Pain Pump for Abdominoplasty: An Alternative to Regional Blocks and Liposomal Bupivacaine. Plast Reconstr Surg. 2015 Nov;136(5):714e–6e. https://doi.org/10.1097/PRS.0000000000001671 PMID:26203979
34. Bagatin D, Bagatin T, Nemrava J, Ivelj MŠ, Deutsch J, Šakić K. Influence of Local Infiltration Analgesia on Postoperative Pain in Abdominoplasty Patients. Acta Clin Croat. 2019 Jun;58 Suppl 1:23–8. https://doi.org/10.20471/acc.2019.58.s1.03 PMID:31741555
35. Kryger ZB, Fine NA, Mustoe TA. The outcome of abdominoplasty performed under conscious sedation: six-year experience in 153 consecutive cases. Plast Reconstr Surg. 2004 May;113(6):1807–17. https://doi.org/10.1097/01.PRS.0000117303.63028.7D PMID:15114149
36. Kakagia DD, Fotiadis S, Tripsiannis G, Tsoutsos D. Postoperative analgesic effect of locally infiltrated levobupivacaine in fleur-de-Lys abdominoplasty. Aesthetic Plast Surg. 2007;31(2):128–32. https://doi.org/10.1007/s00266-006-0187-4 PMID:17205251
37. Stewart D, Yue A, Gianoutsos M. Anchoring of pain pump catheters within the rectus fascia in abdominoplasty. Plast Reconstr Surg. 2008 Apr;121(4):229e–30e. https://doi.org/10.1097/01.prs.0000305390.54251.09 PMID:18349615
38. Mentz HA, Ruiz-Razura A, Newall G, Patronella CK. Use of a regional infusion pump to control postoperative pain after an abdominoplasty. Aesthetic Plast Surg. 2005;29(5):415–21. https://doi.org/10.1007/s00266-005-0062-8 PMID:16177875
39. Bray DA Jr, Nguyen J, Craig J, Cohen BE, Collins DR Jr. Efficacy of a local anesthetic pain pump in abdominoplasty. Plast Reconstr Surg. 2007 Mar;119(3):1054–9. https://doi.org/10.1097/01.prs.0000252536.56982.34 PMID:17312513
40. Michaud AP, Rosenquist RW, Cram AE, Aly AS. An evaluation of epidural analgesia following circumferential belt lipectomy. Plast Reconstr Surg. 2007 Aug;120(2):538–44. https://doi.org/10.1097/01.prs.0000267638.84902.e6 PMID:17632361
41. Alotaibi NN, Ahmad T, Rabah SM, Hamza AM, Mohammad Tafazul S. Evaluation of transversus abdominis plane (TAP) block in lipoabdominoplasty surgical procedure: a comparative study. J Plast Surg Hand Surg. 2021 Aug;55(4):216–9. https://doi.org/10.1080/2000656X.2020.1856676 PMID:33397174
42. Elsafty OMT, Awad HMF, Mahran MGE, Sadek HAF. A Comparative Study between Ultrasound Guided Bilateral Transversus Abdominis Plane block Versus Opioids (Nalbuphine) Analgesia after Abdominoplasty. QJM: An International Journal of Medicine. 2021 Oct 1;114(Supplement_1).
43. Mansour RF, Afifi MA, Abdelghany MS. Transversus Abdominis Plane (TAP) Block: A Comparative Study between Levobupivacaine versus Levobupivacaine plus Ketamine in Abdominoplasty. Pain Res Manag. 2021 Oct;2021:1762853. https://doi.org/10.1155/2021/1762853 PMID:34754346
44. Elsawy A, Abdelhameed S. Bilateral Fascial Plan Block for Post Abdominoplasty Pain Control; Erector Spinae Plane Block Contrasted with Transversus Abdominis One. Al-Azhar International Medical Journal. 2021 Sep;2(9):34–40. https://doi.org/10.21608/aimj.2021.80063.1498.
45. Anwar MU, Rawlins J, Baker P, Fairbrass M, Foo IT. Per-operative infiltration of the rectus sheath in abdominoplasty. Aesthetic Plast Surg. 2008 Jan;32(1):178–178. https://doi.org/10.1007/s00266-007-9013-x PMID:17721716
46. Bjelland TW, Yates TGR, Fagerland MW, Frøyen JK, Lysebråten KR, Spreng UJ. Quadratus lumborum block for postoperative analgesia after full abdominoplasty: a randomized controlled trial. Scand J Pain. 2019;19(4):671-678. Published 2019 May 21. https://doi.org/10.1515/sjpain-2019-0013.
47. Meouchy MG, Awaida CJ, Jabbour HJ, Rayess YA, Jabbour SF, Nasr MW. Ultrasound-Guided Quadratus Lumborum Block for Postoperative Pain in Abdominoplasty: A Randomized Controlled Study. Plast Reconstr Surg. 2021 Apr;147(4):851–9. https://doi.org/10.1097/PRS.0000000000007767 PMID:33710163
48. Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia. 2001 Dec;56(12):1184–8. PMID:11736777
49. Rudkin GE, Gardiner SE, Cooter RD. Bilateral thoracic paravertebral block for abdominoplasty. J Clin Anesth. 2008 Feb;20(1):54–6. https://doi.org/10.1016/j.jclinane.2007.06.020 PMID:18346612

 ORCID
ORCID

 Creative Commons Attribution
Creative Commons Attribution