Leonardo Arce Gálvez1,2,*, David Hernández Abuchaibe1,2, José Luis Cuervo Pulgarín2,5, Claudia Liliana Buitrago Martín3,5
Recibido: 30-11-2023
Aceptado: 02-02-2024
©2024 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 53 Núm. 2 pp. 91-93|https://doi.org/10.25237/revchilanestv53n2-04
PDF|ePub|RIS
Conocimiento actual de los receptores opioides y sus vías de señalización
Abstract
Purpose of review: The purpose of this article is to describe the molecular characteristics and currently conceived mechanisms in the functioning of opioid receptors, opioid drugs are of great interest in the analgesic management of patients with oncologic and non- oncologic pathologies. Recent findings: Opioid receptors are not considered to act as single units, producing a particular effect with their stimulation. They are now perceived as functional assemblies with the possibility of having heterodimeric activation as well as having allosteric characteristics that will modify the response mechanisms. On the other hand, activations of other types of receptors involved in nociception have been determined with the chronic use of drugs acting on opioid receptors. Summary: Endogenous opioid receptors are a group of four receptors that can modify the descending nociceptive pathways modulating pain but that can also activate other types of receptors involved in nociception. Additionally, they are receptors that can be activated jointly or partially and generate different types of responses and side effects.
Resumen
Objetivo de la revisión: El propósito de este artículo es describir las características moleculares y los mecanismos actualmente concebidos en el funcionamiento de los receptores opioides, los fármacos opioides son de gran interés en el tratamiento analgésico de pacientes con patologías oncológicas y no oncológicas. Hallazgos recientes: No se considera que los receptores opioides actúen como unidades individuales, produciendo un efecto particular con su estimulación. Ahora se perciben como conjuntos funcionales con posibilidad de activación heterodimérica, así como con características alostéricas que modificarán los mecanismos de respuesta. Por otra parte, se han determinado activaciones de otros tipos de receptores implicados en la nocicepción con el uso crónico de fármacos que actúan sobre los receptores opioides. Resumen: Los receptores opioides endógenos son un grupo de cuatro receptores que pueden modificar las vías nociceptivas descendentes que modulan el dolor, pero que también pueden activar otros tipos de receptores implicados en la nocicepción. Además, son receptores que pueden activarse conjunta o parcialmente y generar diferentes tipos de respuestas y efectos secundarios.
The endogenous opioid system has four classical receptors that have been extensively studied, including Mu, Delta, Kappa, and NOP (nociceptin opioid peptide receptor)[1].
However, in recent years, advances in the structural understanding of these receptors have shown that there are pathways and modified responses of these receptors to pharmacological stimuli that may explain their side effects, dependence profile and therapeutic response in pain control[2]. The classical opioid pathway generates the activation of G-protein-linked receptors that in turn favor the activation of descending pain modulatory pathways and targets the periaqueductal gray area and the perceptual modification of the somatosensory cortex; however, the molecular process is more complex (Figure 1). On the other hand, opioid drugs are of great interest in the analgesic management of patients with oncologic and non-oncologic pathologies, which generates the need to know their pharmacological mechanisms favoring the best clinical outcomes and their safe use.
We must understand that opioid receptors have special characteristics including allosteric regulation, heteromeric capacities and biased agonisms in some receptors. In the first place, allosteric regulation refers to the binding of a molecule at a specific site that modifies the binding site of another, which at the level of opioid receptors offers the possibility of modifying the response to each receptor as a future therapeutic target. There are therefore positive and negative allosteric modifications were depending on the binding site we will have a greater or lesser opioid agonist response, which is a possible explanation for tolerance and dependence responses. In addition to being a potential pharmacological target where a positive allosteric response could increase the exogenous opioid response[1],[2].
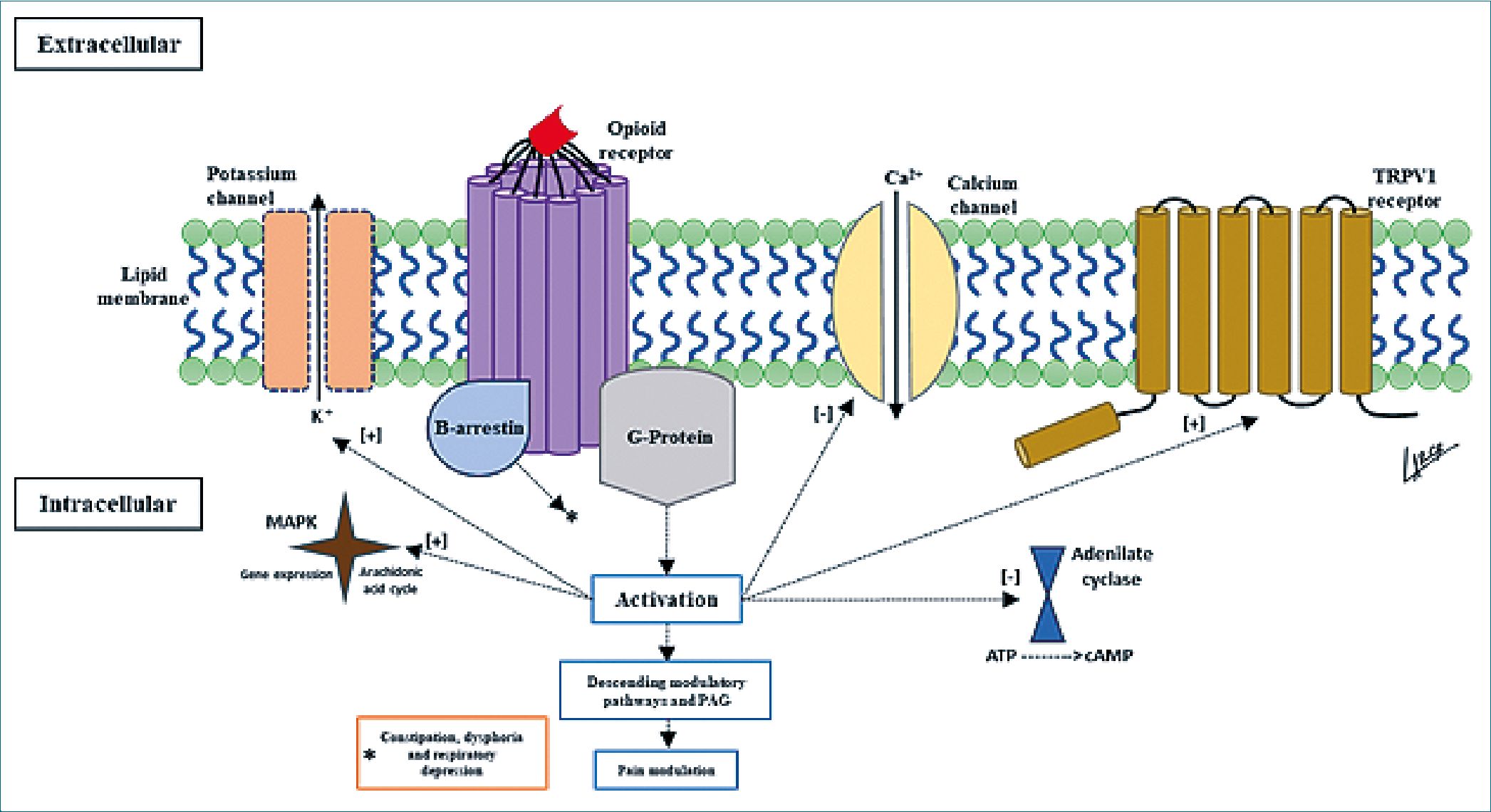
Figure 1. Mechanism of action of opioid receptors.
The heteromeric capacities of opioid receptors are because they work as individual units, and their previous conception is arbitrary. In the activation of opioid receptors there is evidence by bioluminescence of energetic transfer between one receptor and another, the most studied being the MDOR (Mu and Delta heterodimer), these heterodimer receptors are widely expressed in the nervous system and seem to be involved in the response to chronic opioid use, especially morphine. The general function of all types of heterodimer receptors (Mu- Delta, Delta-Kappa, and Mu-Kappa) seems to be the activation of an anti-opioid negative feedback system and the allosteric modification of the other receptors, which would be present in chronic use generating an antinociceptive action in addition to having anxiolytic and antidepressant action as a body defense mechanism to the continuous activation of the endogenous opioid system[1],[3],[4].
Receptor biased agonism speaks of the ability to activate multiple G-protein and beta-arrestin-dependent path- ways[3],[4]. Thus, there are analgesic processes associated with the G-protein pathway and some side effects such as ventilatory suppression and constipation with beta-arrestin, so that G-protein signaling and beta-arrestin recruitment can predict the outcome of opioid medication use[4],[5]. Little is currently known about the biased activation pathway but it is thought to be an allosteric response where for example binding and further mutation of the sodium allosteric portion of the Mu receptor, where decreasing sodium increases beta-arrestin recruitment, which would mean at the Mu receptor an increase in ventilatory pressure and constipation and dysphoric events at the Kappa receptor, as the analgesic effects are mediated by G-protein signaling[2],[4],[5]
Finally, it is worth highlighting the reciprocal activation between Mu opioid receptors and TRPV1 (transient receptor potential vanilloid 1) receptors, which are involved in cutaneous thermal perception and are a current therapeutic target. The reciprocal activation of TRPV1 receptors could explain the events of opioid-induced hyperalgesia, given the high nociceptive stimulus that would be generated. It is also known that in cases of chronic opioid use there is a modification in the inverse direction where the reciprocal activation of TRPV1 generates a decrease in the Mu receptors’ antinociceptive action, also increasing the amount of substance P and calcitonin gene-related peptide (CGRP)[6].
The concept of endogenous opioid receptors as independent functional units has been reevaluated; considering everything explained above, it should be taken into account that the use of opioid analgesic medications leads to the activation of multiple receptors, their clinical response will depend on the allosteric conditions of each medication and of each patient, besides that biased activations can alter the expected therapeutic response, on the other hand, the reciprocal activation of other pro nociceptive receptors, especially in the chronic use of these medications can favor additional pain conditions that can overlap and generate complex cases. This is a summary that invites us to know and investigate more and more this type of receptors and possible pathways involved in their responses.
Funding: The authors did not receive funding to carry out this study.
Conflict of interest: We declare no conflict of interest.
-
References
1. Regueras E, Torres LM, Velazquez I. Nuevas estrategias y generaciones de analgésicos opioides: ¿qué se está investigando? Multidisciplinary Pain Journal; 2022. https://doi.org/10.20986/mpj.2022.1030/2022.
2. Valentino RJ, Volkow ND. Untangling the complexity of opioid receptor function. Neuropsychopharmacology. 2018 Dec;43(13):2514–20. https://doi.org/10.1038/s41386-018-0225-3 PMID:30250308
3. Pardo, M., ed. Miller’s Basics of Anesthesia. 8th ed. July 5, 2022.
4. Wang Y, Zhuang Y, DiBerto JF, Zhou XE, Schmitz GP, Yuan Q, et al. Structures of the entire human opioid receptor family. Cell. 2023 Jan;186(2):413–427.e17. https://doi.org/10.1016/j.cell.2022.12.026 PMID:36638794
5. Malcolm NJ, Palkovic B, Sprague DJ, Calkins MM, Lanham JK, Halberstadt AL, et al. Mu-opioid receptor selective superagonists produce prolonged respiratory depression. iScience. 2023 Jun;26(7):107121. https://doi.org/10.1016/j.isci.2023.107121 PMID:37416459
6. Bao Y, Gao Y, Yang L, Kong X, Yu J, Hou W, et al. The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence. Channels (Austin). 2015;9(5):235–43. https://doi.org/10.1080/19336950.2015.1069450 PMID:26176938

 ORCID
ORCID