Esam Hamed1, Rasha Hamed*, Amal Mubarak2, Hamdi Abbas3
Recibido: 29-09-2024
Aceptado: 24-09-2024
©2025 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 54 Núm. 3 pp. 288-293|https://doi.org/10.25237/revchilanestv54n3-10
PDF|ePub|RIS
Abstract
Background: Single-injection peripheral nerve block (PNB) is commonly used for perioperative analgesia and anesthesia. Three approaches for extending the duration of PNB include continuous PNB with catheter-based techniques, novel local anesthetics delivery systems and addition of novel adjuvants to local anesthetics. Methods: 85 out of the 90 patients who completed the study. Patients were randomly allocated into one of two groups: Group (DS) 42 patients will receive 20 ml mixture of 0.25% bupivacaine and 4 mg dexamethasone diluted in 2ml normal saline. Group (DS) 42 patients will receive 20 ml mixture of 0.25% bupivacaine and 0.5 Mcg/kg dexmedetomidine diluted in 2ml normal saline. Results: Significantly lower postoperative VAS scores found in dexmedetomidine group in comparison to dexamethasone group. Also, duration of analgesia was significantly longer in dexmedetomidine group in comparison to dexamethasone group. Conclusion: Dexmedetomidine addition to isobaric bupivacaine in ultrasound guided adductor canal block is more effective than dexamethasone in prolonging postoperative analgesia duration and postoperative nalbuphine consumption.
Resumen
El bloqueo de nervios periféricos (BNP) de inyección única se usa comúnmente para analgesia y anestesia perioperatoria. Tres enfoques para extender la duración de la BNP incluyen la BNP continua con técnicas basadas en catéter, nuevos sistemas de administración de anestésicos locales y la adición de nuevos adyuvantes a los anestésicos locales. Métodos: 85 de los 90 pacientes que completaron el estudio. Los pacientes fueron asignados aleatoriamente a uno de dos grupos: Los pacientes del grupo (DS) 42 recibieron 20 ml de una mezcla de bupivacaína al 0,25% y 4 mg de dexametasona diluida en 2 ml de solución salina normal. Los pacientes del grupo (DS) 42 recibieron 20 ml de una mezcla de bupivacaína al 0,25% y 0,5 mcg/kg de dexmedetomidina diluida en 2 ml de solución salina normal. Resultados: Se encontraron puntuaciones EVA posoperatorias significativamente más bajas en el grupo de dexmedetomidina en comparación con el grupo de dexametasona. Además, la duración de la analgesia fue significativamente mayor en el grupo de dexmedetomidina en comparación con el grupo de dexametasona. Conclusión: La adición de dexmedetomidina a bupivacaína isobárica en el bloqueo del canal aductor guiado por ecografía es más eficaz que la dexametasona para prolongar la duración de la analgesia y el consumo posoperatorios de nalbufina.
-
Introduction
Single-injection peripheral nerve block (PNB) is commonly used for perioperative analgesia and anesthesia[1]. Although PNB are beneficial for improved early postoperative pain management, it is often insufficient, as postoperative pain can persist for several days. The aim of prolonging the duration of PNB to treat postoperative pain is a key issue in regional anesthesia. Three approaches for extending the duration of PNB include continuous PNB with catheter-based techniques, novel local anesthetics delivery systems and addition of novel adjuvants to local anesthetics[2]. Adjuvants that are frequently added to local anesthetics to prolong analgesia following single-injection PNB include epinephrine, opioids, tramadol, ketamine, midazolam, magnesium, clonidine, dexmedetomidine and dexamethasone, but often with limited success and unproven safety[3]-[4]. Studies of perineural buprenorphine, dexamethasone and dexmedetomidine have most consistently demonstrated prolongation of PNB[5]. Dexamethasone is a potent long-acting steroid that has shown efficacy as an adjuvant to local anesthetics in various studies[6]-[7]. Dexmedetomidine enhances PNB when added to local anesthetics, providing better quality of anesthesia as well as postoperative analgesia[8]-[9]. The mechanism by which dexamethasone and dexmedetomidine prolong the duration of local anesthetics are not completely understood and may arise from various factors. Both dexamethasone and dexmedetomidine can reduce local inflammation and prolong the duration of nerve block through vasoconstriction by maintaining the local concentration of the local anesthetic[10]-[11]. Vasoconstriction also inhibits the nociceptive impulse transmission along myelinated C fibers[12]. Possible mechanisms of dexmedetomidine in prolonging the duration of nerve blocks may also include the inhibition of the hyperpolarization- activated cation current. 13 Some research suggests that dexmedetomidine may provide local anesthetic action that blocks the conduction of nerve signals through C and A3 fibers, not through a2 action, and may stimulate the release of enkephalin-like substances at peripheral sites[14].
Due to the different mechanisms of action, we aimed to compare the efficacy of dexamethasone and dexmedetomidine in prolonging analgesia duration after adductor canal block in patients undergoing knee arthroscope.
-
Patient and Methods
This is a prospective randomized double-blind study that was carried out at Assuit University hospitals, after obtaining local ethical approval (IRB:17101243). Clinical trial registration NCT06527976. 85 out of the 90 patients completed the study. Patients were randomly allocated into two groups:
Dexmedetomidine Group 42 patients will receive 20 ml mixture of 0.25% bupivacaine and 4 mg dexamethasone diluted in 2ml normal saline.
Dexamethasone Group 43 patients will receive 20 ml mixture of 0.25% bupivacaine and 0.5 Mcg/kg dexmedetomidine diluted in 2ml normal saline.
Randomization was done using computer generated numbers in 1:1 ratio. Study drugs will be prepared by an anesthetist other than the anesthetist responsible for giving the block.
Patients, anesthetist, and data collector will all be blind to the study groups. Inclusion criteria: Patients scheduled for elective kne arthroscopy with American Society of Anesthesiologists (ASA) physical status I -II, mentally competent and able to give informed written consent for enrollment in the study. Patients excluded if refused to consent, ASA III and VI, patient with coagulopathy and BMI of 40 or more.
-
Anesthesia Technique
The standard ASA monitors applied to the patient then the patient positioned in the sitting position and the back sterilized by betadine antiseptic solution and allowed to dry before performing the puncture. 12.5 mg of hyperbaric bupivacaine given at the L3/4 interspaces (alternatively at the L2/3 or L4/5 interspaces) for spinal anesthesia.
-
Block Performance
Block done immediately at the end of the operation. All blocks were done under ultrasound guidance, GE LOGIQ-6 machine was used with a high frequency linear (HFL) transducer (6-13 MHZ ). In short axis view of the thigh, the sartorius muscle which descends in a lateral to the medial direction across the anterior thigh identified forming the “roof” of the adductor canal in the lower half of the thigh. The muscle appears as a trapezoid shape beneath the subcutaneous layer of adipose tissue. The sides of the adductor canal formed by the vastus me- dialis laterally and the adductor longus and magnus medially. The saphenous nerve identified as a small, round, hyperechoic structure anterior to the artery. The femoral vein accompanies the artery and saphenous nerve, which all can be identified at a depth of 2-3 cm. The needle introduced from lateral to medial in an in-plane technique. 2-3 ml normal saline used to verify correct placement of the needle in the vicinity of the saphenous nerve in the adductor canal, then a bolus of 20 ml of local anesthetic mixture is injected.
-
Assessment parameters
1. VAS scores assessed by well-trained anesthesia nurse in PACU at 4, 6, 12, 18 and 24 hours postoperative.
2. Post block hemodynamics.
3. Time to first analgesic request; it is the first time the patient ask for analgesia.
4. Opioid consumption for the first 24 hours after surgery.
-
Sample size
The sample size calculated using G*Power analysis. Depending on results of a previous study[15]. mean VAS score after 4h of perineural dexmedetomidine was 2.67, if the SD ± 1.2, 40 patients in each group are required to detect a difference of one in VAS between the two groups, with an alpha level of 0.05, a beta level of 0.1 and 95% power. To compensate for dropouts, 90 patients were enrolled.
Statistical analysis
Data entry and data analysis done using SPSS version 22 (Statistical Package for Social Science). Data presented as number, Percentage, mean, median and standard deviation. Chi
square used to compare between qualitative variables. Independent samples t-test used to compare quantitative variables between groups in case of parametric data and Mann-Whitney test Used for non-parametric data. Repeated measure analysis for repeated measure comparison (VAS score) between two groups.
-
Results
Ninety patients scheduled for elective knee arthroscope under spinal anesthesia. They met the eligibility criteria and were randomly allocated to receive ultrasound guided adductor canal block immediately after the end of surgical intervention either with dexmedetomidine or dexamethasone addition to bupivacaine local anesthetic. 85 patients completed the study (Figure 1), 42 in the dexmedetomidine and 43 in the dexamethasone group. No significant difference found in the demographic data or surgical characteristics (Table1). A significant difference found in the hemodynamics from 30 minutes to 120 minutes after block, but this was of no clinical importance as all values were in the normal range (Figure 2). Significantly lower postoperative VAS scores found in dexmedetomidine group in comparison to dexamethasone group (Table 2 and Figure 3). Furthermore, duration of analgesia was significantly longer in dexmedetomidine group in comparison to dexamethasone group, as time to first analgesia request was 15h ± 4 in dexmedetomidine group versus 12h ± 3; P = 0.002 in dexamethasone group (Table 3). Also, amount of postoperative nalbuphine consumption was significantly lower in dexmedetomidine group (5 mg ± 3) in comparison to dexamethasone group (9 mg ± 4) with P < 0.001.
-
Discussion
The present study aimed to compare the effect of adding dexamethasone or dexmedetomidine to bupivacaine to improve the efficacy of ACB in patients undergoing knee arthroscope. Dexmedetomidine found to be more effective than dexamethasone in prolonging the analgesic effect of bupiva- caine local anesthetic and minimizing the requirement of opioid analgesia. There is a rising focus on the advantages of peripheral nerve blocks in ambulatory and orthopedic facilities. Various additives are incorporated to enhance the efficacy of nerve blocks in prolonging analgesia duration. Alpha-2 agonists such as dexmedetomidine and glucocorticoids such as dexamethasone are famous additives with promising effects[16].
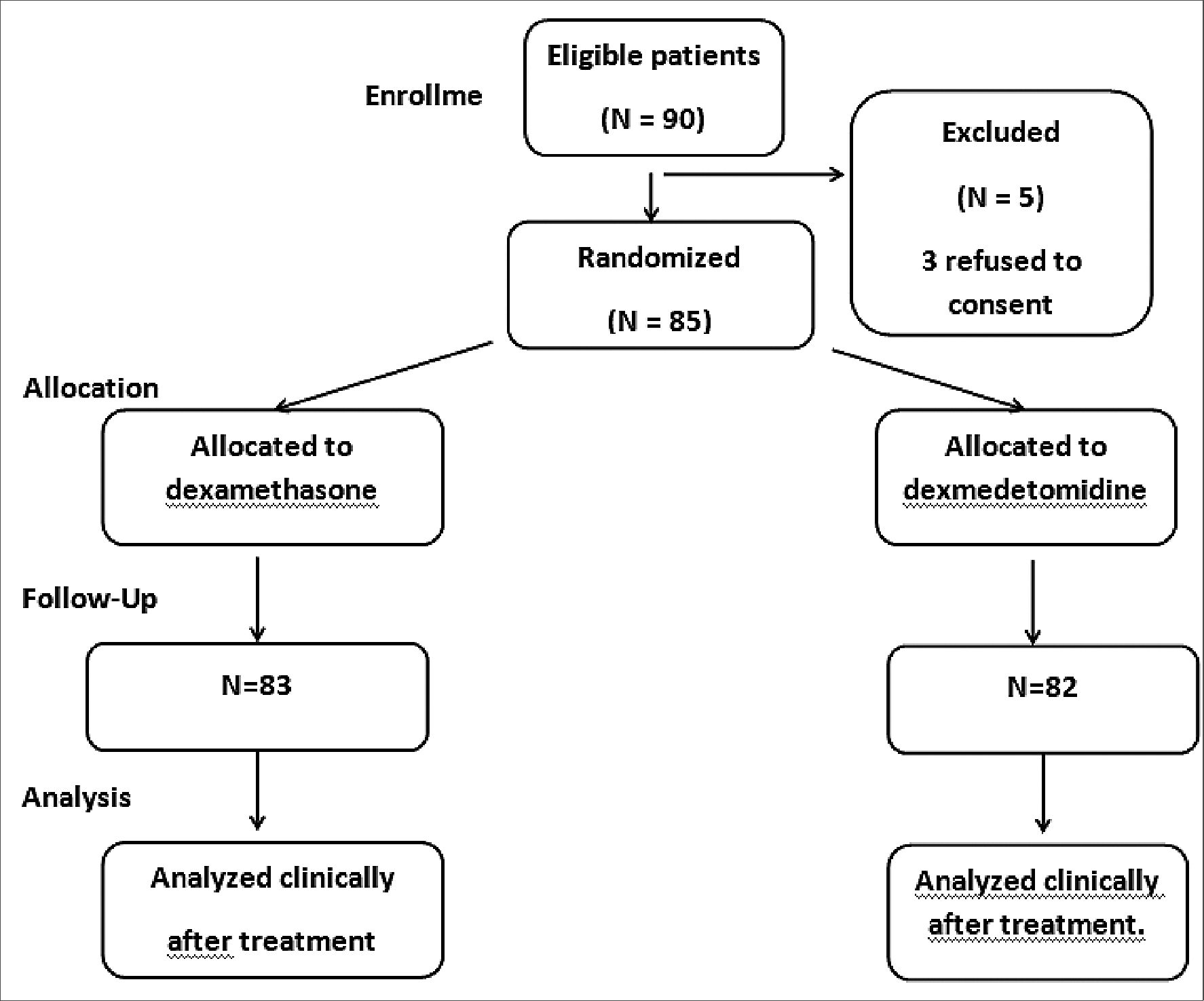
Figure 1. Flow chart.
Table 1. Demographic data of the studied groups
| Dexamethasone group (n = 45) | Dexmedetomidine group (n = 45) | P value | |
| Age (years) | 41± 8 | 39 ± 8 | 0.4 |
| Sex | |||
| Male | 20 | 24 | 0.45 |
| Female | 22 | 19 | |
| Body mass index (kg/m2) | 27.35 ± 3.95 | 26.70 ± 4.34 | 0.2 |
| ASA class | |||
| Class-I | 21 | 27 | 0.3 |
| Class-II | 21 | 16 | |
| Surgery duration | 56 ± 13 | 59 ± 10 | 0.9 |
Data expressed as frequency (percentage), mean± (SD); P value was significant if < 0.05; ASA: American society of anesthesiologists; BMI: body mass index.
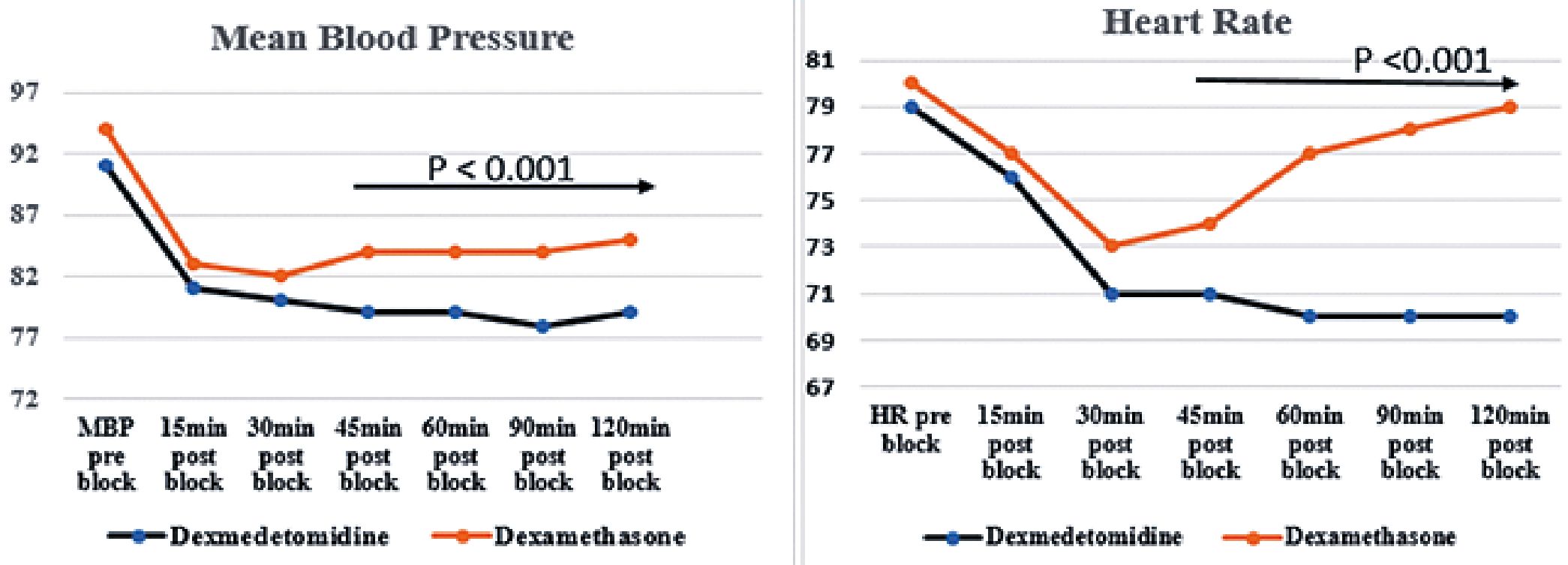
Figure 2. Hemodynamics between the study groups.
Table 2. VAS score between groups
| Time of assessment | Dexmedetomedine | Dexamethasone | P value |
| 4 h-Post block | 0 ± 0 | 0.4 ± 0.5 | < 0.001 |
| 6 h-Post-block | 0.2 ± 0.4 | 1.8 ± 1.1 | < 0.001 |
| 12 h-Post-block | 1.1 ± 0.7 | 2 ± 1.7 | 0.02 |
| 18 h-Post-block | 0.9 ± 1 | 1.5 ± 1.3 | 0.01 |
| 24 h post-block | 0.9 ±1.2 | 1.4 ± 1 | < 0.001 |
Table 3. Time to 1st analgesia and 24 hour nalbuphine consumption
| Dexmedetomidine | Dexamethasone | P value | |
| 1st analgesic request (hour) | 15 ± 4 | 12 ± 4 | 0.002 |
| 24 h nalbuphine consumption | 5 ± 3 | 9 ± 4 | < 0.001 |
Date expressed as mean (SD). P: value was significant if < 0.01.
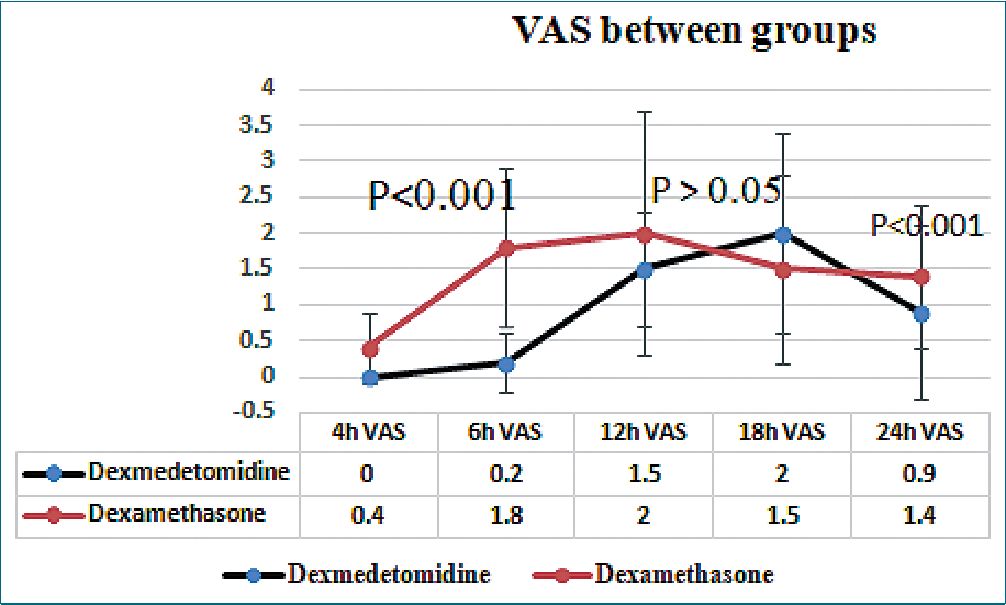
Figure 3. VAS scores between the study groups.
To best of our search we did not found studies compared dexamethasone and dexmedetomidine in adductor canal block, but comparison was carried in other blocks. In accordance with our study in: Gao et al., compared effect of dexamethasone versus dexmedetomidine in ultrasound guided erector spinae block for video assisted thoracoscope, they reported that dexmedetomidine is more superior to dexamethasone in prolonging sensory block duration and analgesia time. Also, dexmedetomidine was more efficient in reducing postoperative opioid consumption and shortening hospital stay[17). Hamada et al., also compared the effect of both drugs in supraclavicular brachial plexus block, they found that dexmedetomidine provided longer analgesia duration than dexamethasone[18].
In a more recent study, Nagraju et al. conducted a comparative study between both drugs in supraclavicular brachial plexus block, they also found that dexmedetomidine is more efficient than dexamethasone in prolonging analgesia duration and minimizing postoperative opioid consumption[19].
Verma et al., observed a prolonged block with dexmedetomidine when compared with dexamethasone as adjuvant with 0.5% ropivacaine in supraclavicular block during elective upper limb surgical procedures[20). Kaur et al., compared the effects of 8 mg of dexamethasone with 50 pg of dexmedetomidine as an adjuvant with a mixture of 20 ml of 2% lignocaine with adrenaline and 18 ml of 0.5% bupivacaine in supraclavicular block[16]. They found that dexmedetomidine prolonged the block when compared with dexamethasone[21].
Mohammed et al., also compared both drugs in patients undergoing abdominal hysterectomy received erector spinae block, they found that dexmedetomidine is more efficacious than dexamethasone in prolonging postoperative analgesia decreasing total nalbuphine consumption[22].
In contrast the present study, Albrecht et al., in their meta-analysis they concluded that both drugs could prolong analgesia duration after supraclavicular brachial plexus block, however their meta-analysis was an indirect meta-analysis that generated low quality evidence that dexamethasone is a superior adjunct; it prolongs analgesia by a statistically significant increase, equivalent to 2.5 hours more than dexmedetomidine[23]. In a direct meta-analysis carried by Song et al., despite they noticed a longer duration of sensory block onset and analgesia duration with dexmedetomidine, there was no difference between both drugs in analgesia duration or postoperative opioid consumption in the statistical analysis results[24]. This is a unicentric trial which limits the study, further studies are encouraged to support the results.
-
Conclusion
Dexmedetomidine addition to isobaric bupivacaine in ultrasound guided adductor canal block is more effective than dexamethasone in prolonging postoperative analgesia duration and postoperative nalbuphine consumption.
Conflicts of interest: The authors have no conflicts of interest. No ghost authors.
-
References
1. Fredrickson Fanzca MJ, Danesh-Clough TK, White R. Adjuvant dexamethasone for bupivacaine sciatic and ankle blocks: results from 2 randomized placebo-controlled trials. Reg Anesth Pain Med. 2013;38(4):300–7. https://doi.org/10.1097/AAP.0b013e318292c121 PMID:23698496
2. King CH, Beutler SS, Kaye AD, Urman RD. Pharmacologic properties of novel local anesthetic agents in anesthesia practice. Anesthesiol Clin. 2017 Jun;35(2):315–25. https://doi.org/10.1016/j.anclin.2017.01.019 PMID:28526152
3. Bailard NS, Ortiz J, Flores RA. Additives to local anesthetics for peripheral nerve blocks: Evidence, limitations, and recommendations. Am J Health Syst Pharm. 2014 Mar;71(5):373–85. https://doi.org/10.2146/ajhp130336 PMID:24534592
4. Knight JB, Schott NJ, Kentor ML, Williams BA. Neurotoxicity of common peripheral nerve block adjuvants. Curr Opin Anaesthesiol. 2015 Oct;28(5):598–604. https://doi.org/10.1097/ACO.0000000000000222 PMID:26207854
5. Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS One. 2015 Sep;10(9):e0137312. https://doi.org/10.1371/journal.pone.0137312 PMID:26355598
6. Bjørn S, Linde F, Nielsen KK, Børglum J, Hauritz RW, Bendtsen TF. Effect of perineural dexamethasone on the duration of single injection saphenous nerve block for analgesia after major ankle surgery: a randomized, controlled study. Reg Anesth Pain Med. 2017;42(2):210–6. https://doi.org/10.1097/AAP.0000000000000538 PMID:28033159
7. Parrington SJ, O’Donnell D, Chan VW, Brown-Shreves D, Subramanyam R, Qu M, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med. 2010;35(5):422–6. https://doi.org/10.1097/AAP.0b013e3181e85eb9 PMID:20814282
8. Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39(1):37–47. https://doi.org/10.1097/AAP.0000000000000033 PMID:24317234
9. Hussain N, Grzywacz VP, Ferreri CA, Atrey A, Banfield L, Shaparin N, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: a systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42(2):184–96. https://doi.org/10.1097/AAP.0000000000000564 PMID:28178091
10. Hu J, Vacas S, Feng X, Lutrin D, Uchida Y, Lai IK, et al. Dexmedetomidine prevents cognitive decline by enhancing resolution of high mobility group box 1 protein-induced inflammation through a Vagomimetic action in mice. Anesthesiology. 2018 May;128(5):921–31. https://doi.org/10.1097/ALN.0000000000002038 PMID:29252509
11. Marks R, Barlow JW, Funder JW. Steroid-induced vasoconstriction: glucocorticoid antagonist studies. J Clin Endocrinol Metab. 1982 May;54(5):1075–7. https://doi.org/10.1210/jcem-54-5-1075 PMID:7061698
12. Johansson A, Hao J, Sjölund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibres. Acta Anaesthesiol Scand. 1990 Jul;34(5):335–8. https://doi.org/10.1111/j.1399-6576.1990.tb03097.x PMID:2167604
13. Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011 Oct;115(4):836–43. https://doi.org/10.1097/ALN.0b013e318221fcc9 PMID:21666435
14. Nakamura M, Ferreira SH. Peripheral analgesic action of clonidine: mediation by release of endogenous enkephalin-like substances. Eur J Pharmacol. 1988 Feb;146(2-3):223–8. https://doi.org/10.1016/0014-2999(88)90296-8 PMID:3163552
15. Ahuja V, Thapa D, Chander A, Gombar S, Gupta R, Gupta S. Role of dexmedetomidine as adjuvant in postoperative sciatic popliteal and adductor canal analgesia in trauma patients: a randomized controlled trial. the Korean journal of pain. 2020 Apr 1;33(2):166-75.
16. Margulis R, Francis J, Tischenkel B, Bromberg A, Pedulla D, Grtisenko K, et al. Comparison of dexmedetomidine and dexamethasone as adjuvants to ultra-sound guided interscalene block in arthroscopic shoulder surgery: a double-blinded randomized placebo-controlled study. Anesth Pain Med. 2021 Jul;11(3):e117020. https://doi.org/10.5812/aapm.117020 PMID:34540645
17. Gao Z, Xiao Y, Wang Q, Li Y. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Ann Transl Med. 2019 Nov;7(22):668. https://doi.org/10.21037/atm.2019.10.74 PMID:31930069
18. Hamada MH, Ibrahim WM, Ashiry MA. Comparative study between Dexmedetomidine and Dexamethasone as an adjuvant to Bupivacaine in ultrasound guided supraclavicular brachial plexus block in upper limb surgeries. Egypt J Hosp Med. 2019 Apr;75(6):3060–9. https://doi.org/10.21608/ejhm.2019.34218.
19. A N, Sahu L, Das S, Muni M. Comparative evaluation of dexmedetomidine and dexamethasone as adjuvants in supraclavicular brachial plexus block. Cureus. 2023 May;15(5):e38775. https://doi.org/10.7759/cureus.38775 PMID:37303417
20. Verma NK, Ranjan A. A clinical comparison of dexmedetomidine and dexamethasone as adjuvant to ropivacaine in supra- clavicular brachial plexus blocks for upper arm surgeries. Int J Adv Res Bio Sci. 2016;3:56–61.
21. Kaur M, Lakhani A, Hashia AM. Comparative study between dexamethasone and dexmedetomidine in supraclavicular block. Int J Adv Med. 2018;5(1):57–61. https://doi.org/10.18203/2349-3933.ijam20175878.
22. Mohammed Ali DS, Salama AM, Abaza KA, Ahmed FM. Dexamethasone versus Dexmedetomidine as Adjuvant to Bupivacaine in Ultrasound Guided Erector Spinae Plane Block for Analgesia in Total Abdominal Hysterectomy. Egypt J Hosp Med. 2022 Jul;88(1):4051–6. https://doi.org/10.21608/ejhm.2022.254083.
23. Albrecht E, Vorobeichik L, Jacot-Guillarmod A, Fournier N, Abdallah FW. Dexamethasone is superior to dexmedetomidine as a perineural adjunct for supraclavicular brachial plexus block: systematic review and indirect meta-analysis. Anesth Analg. 2019 Mar;128(3):543–54. https://doi.org/10.1213/ANE.0000000000003860 PMID:30303864
24. Song ZG, Pang SY, Wang GY, Zhang Z. Comparison of postoperative analgesic effects in response to either dexamethasone or dexmedetomidine as local anesthetic adjuvants: a systematic review and meta-analysis of randomized controlled trials. J Anesth. 2021 Apr;35(2):270–87. https://doi.org/10.1007/s00540-021-02895-y PMID:33515302

 ORCID
ORCID

