Marisol Zuluaga Giraldo1,2,3,*, Paulina Castro Echavarría4, Simón Galindo Zuluaga5
Recibido: 25-03-2023
Aceptado: 12-04-2023
©2023 El(los) Autor(es) – Esta publicación es Órgano oficial de la Sociedad de Anestesiología de Chile
Revista Chilena de Anestesia Vol. 52 Núm. 4 pp. 409-414|https://doi.org/10.25237/revchilanestv52n04-14
PDF|ePub|RIS
Laringectomía total en un neonato con sarcoma laringeo congénito: Manejo perioperatorio; reporte de un caso y revisión de la literatura
Abstract
Newborn airway related diseases are one of the biggest challenges that an anesthesiologist can face during his medical practice. This case report describes a complete airway obstruction in a neonate with a laryngeal sarcoma who underwent various diagnostic procedures, finally receiving a total laryngectomy as part of his oncological treatment. We document the perioperative care during the laryngectomy, a procedure that has not yet been reported in literature to the date. We share our experience on how to confront such challenge and review the current literature on laryngeal cancer in the pediatric population.
Resumen
La patología relacionada con la vía aérea en el recién nacido es uno de los grandes retos a los que se enfrenta un anestesiólogo; en este reporte de caso se presenta un neonato con obstrucción completa de la vía aérea por un fibrosarcoma laríngeo sometido a varios procedimientos diagnósticos bajo anestesia general y finalmente se le realiza una laringectomia total. Como parte de su manejo oncológico. Se documenta el manejo perioperatorio de la laringectomia; procedimiento hasta el momento no reportado en neonatos, se comparte la experiencia de enfrentar un reto complejo y se hace una revisión de literatura del cáncer laríngeo en la población pediátrica.
-
Introduction
Globally, head and neck cancers are reported to be a relatively frequent condition between the fifth and seventh decades of life, being rare in those under 40 years of age. Its incidence is 650,000 cases per year with an annual death rate of 300,000 for the adult patient[1]. However, laryngeal carcinoma is an extremely rare disease among the pediatric ages. The first case was reported in 1868 in a 3-year-old child.
Head and neck malignant neoplasms represent 2 to 10% of solid tumors during the pediatric ages but laryngeal carcinoma is as low as 0.1% of them all. Most commonly described laryngeal neoplasms in the pediatric population are squamous cell carcinoma and rhabdomyosarcoma. Other malignant laryngeal tumors include minor salivary gland carcinoma, primitive neuro- ectodermal tumors (PNET) and metastatic lesions.
First reports in literature describe that Squamous cell carcinoma develops when a benign laryngeal papilloma undergoes radiation[2]. There are few reported cases for mesenchymal cell tumors, being rhabdomyosarcoma the most frequently described, followed by synovial sarcoma, Ewing’s sarcoma, fibrosarcoma and mixed sarcoma. In 1941, Dr. Rigby and Dr. Holinger from the Children Memorial Hospital in Chicago re- ported the one and only neonatal laryngeal fibrosarcoma described to date. There’s no consensus on how to treat the pathology in an adequate manner[3],[4],[5].
We report the perioperative management of a neonate with a congenital laryngeal fibrosarcoma who was taken to a total laryngectomy as part of the global management of his disease and review laryngeal cancer in the pediatric population. There are no current available reports of the perioperative management for a total laryngectomy in neonates.
Table 1. The pre-surgical admission reported
| Parameter | Result |
| Hemoglobin g/dL | 10 |
| Hematocrit % | 30 |
| Platelets mm3 | 315,000 |
| Coagulation studies | TP: 12.7/11.4 INR:1.1 TPT:34.57/31.5 Ratio 1:1 |
| Blood chemistry meq/L | Calcium: 9.8 Chloride: 102 Sodium: 134 Potassium: 4.46 Magnesium: 1.85 |
| Creatinine mg/dL | 0.47 |
-
Case report
A 37 + 4 weeks natural born neonate with normal pre-natal conditions debuted with severe stridor at his fourth day of life. In a fiber nasolaryngoscopy, a tumor occluding almost 100% of the airway in the glottic and supra glottic region was found. Biopsies were taken in a laryngeal micro-endoscopy, performing then a partial resection of the tumor. Later, the newborn presented again with a complete airway obstruction, needing a new orotracheal intubation. Biopsies, although not concluding, reported a fusocellular tumor compatible with a rhabdomyosar- coma. Finally, the patient was transferred to our institution for further investigations and a multidisciplinary management.
An initial neck/thorax CT reported a diminished airway (99%) caliber in the supra, epi and glottic regions. Supraglottic structures were not easily identified and the vallecules and piriform sinuses were completely obliterated. The airway began to have a near normal caliber at the height of the thyroid gland. Simple and contrasted MRI described a tumor-like mass involv- ing the laryngeal region in the supraglottic, glottic and subglottic region, with no extralaryngeal extension.
The case was then discussed in a medical staff, in which the decision was to perform a laryngeal microsurgery to take new biopsies. The samples showed a congenital laryngeal fibrosar- coma, so in a last meeting the total laryngectomy was decided. The pre-anesthetic evaluation was done for total laryngectomy. We had a male patient of 1 month old weighing 4,100 grams. Parents were explained the scheduled surgical procedure, risks and complications, they understood, accepted and gave their consent for their child’s surgery and blood transfusion if necessary. A unit of fresh and leuko reduced red blood cells was reserved. Instructions about enteral route were given (Table 1).
The surgical procedure was performed with basic ASA monitoring, oropharyngeal temperature, diuresis, and invasive arterial pressure (radial artery). The intravascular volume and allowable blood loss were calculated (320 and 70 ml respectively) according to patient’s weight and age.
Two peripheral venous lines were cannulated with a 24 catheter in addition to a 4 Fr bilumen central venous catheter. Temperature maintenance was carried out with a convective air blanket, sterile plastic covering the entire surface of the patient, warm intravenous fluids, and a respiratory circuit with warm and humid air.
The airway was initially patent through the orotracheal route and later through a surgical stoma at the tracheal level with a number 3.5 tube with balloon. The ventilatory strategy used was pressure-controlled mechanical ventilation with guar- anteed volume (autoflow) with a FI02 between 60%-70%. The anesthetic maintenance was done with Sevoflurane (1 MAC) and fentanyl infusion (3 mcg/kg/h) and ketamine (30 mcg/kg/ min). Maintenance of intravenous fluids was performed with dextrose at 10%, replacement of blood losses initially with balanced solution (plasmalyte) and later with red blood cells when the permissible blood losses were exceeded. Maintenance of muscle relaxation was performed with rocuronium (0.6 mg/kg) and antibiotic prophylaxis with ampicillin sulbactam (50 mg/kg). During the intraoperative period, it was necessary to administer calcium gluconate due to hypocalcemia evidenced in the arterial gases. Calcium gluconate 100 mg/kg was administered by the central line.
In the intraoperative period, due to the small size of the structures and due to surgical manipulation, there was inadvertent mobilization of the 3.5 tube located in the tracheal stoma towards the outside or towards the right main bronchus on several occasions. Additionally, at the beginning of the surgical procedure, an increase in the difference of PaCO2 and ETCO2 was detected early in the initial arterial gases due to the dead space produced by the tube located in the tracheal stoma, which was corrected.
At the end of the surgical procedure, the tracheal tube was replaced by a Shiley cannula tracheostomy (4.0), verifying ad- equate bilateral thoracic expansion and adequate oxygenation and ventilation.
The anesthetic time was 5 hours 30 minutes, and the surgical time was 4 hours 30 minutes. There were no surgical or anesthetic complications during laryngectomy. Intra-operative bleeding was calculated as 120 ml (higher than the calculated allowable blood losses). A total of 450 ml of crystalloids were administered (plasmalyte plus metabolic flow with dextrose) and 60 ml of red blood cells (15 ml/kg). The patient was transferred to the neonatal intensive care unit with a patent tracheostomy, he was hemodynamically stable with adequate parameters of oxygenation, ventilation, and perfusion. Final arterial gases reported lactic acid of 1.4 mmol/L, ions and hemoglobin were normal for his age. The resected larynx was sent for a pathological anatomy study. The post-operative recovery was very satisfactory, and he completed the management of laryn- geal cancer with chemotherapy indicated by the oncology service (Figure 1-2-3).
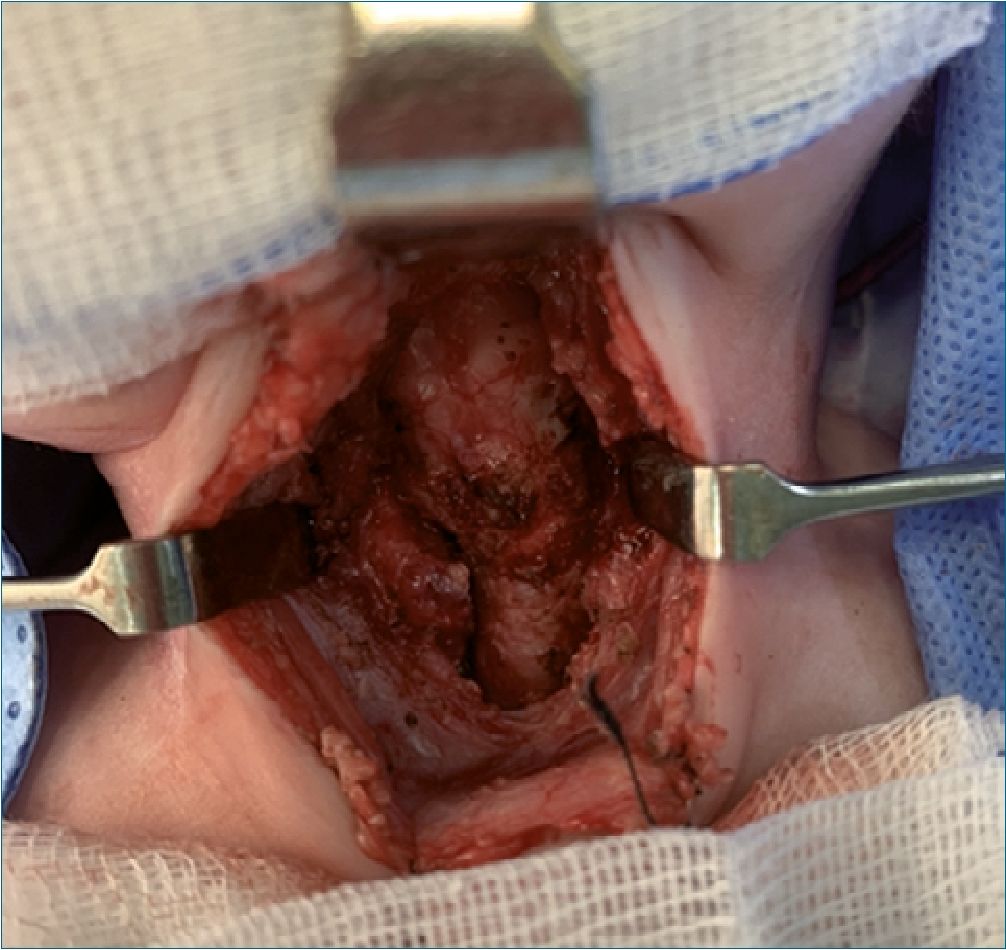
Figure 1. Larynx dissection.
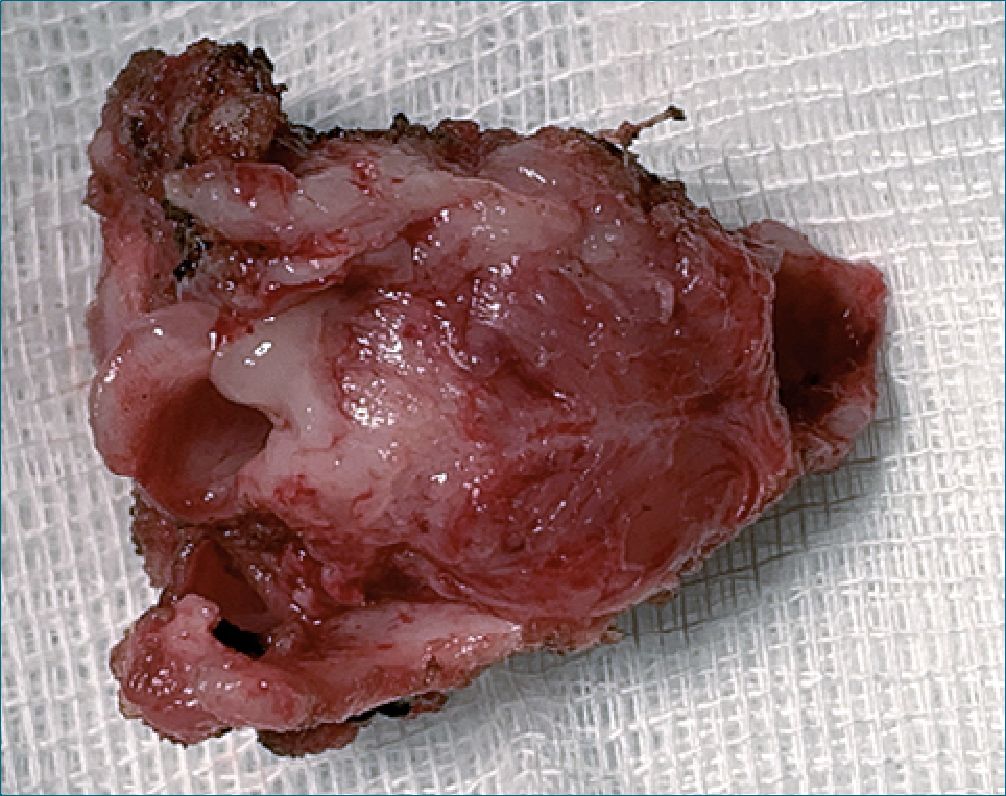
Figure 3. Larynx.
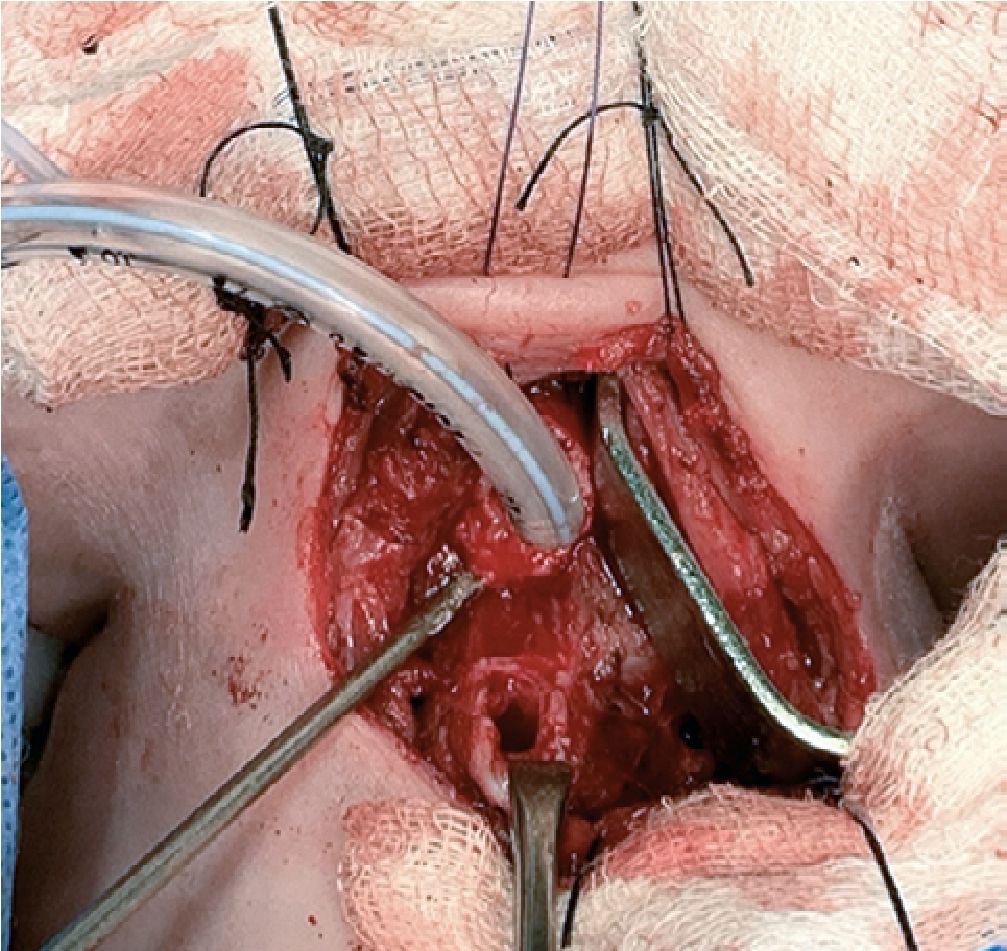
Figure 2. Orotraqueal tube in traqueal stoma.
-
Discussion
In children and adolescents, malignant tumors of the larynx are rare. Institutional reviews and case reports in patients
under 15 years of age have shown that laryngeal carcinoma accounts for less than 0.1% of malignant head and neck tu- mors[6],[7],[8],[9],[10].
Recent studies indicate that many malignant tumors today are derived from mesodermal tissue, in contrast to initial reports in the literature of squamous cell tumors related to irradiation of benign lesions such as juvenile laryngeal papillomatosis.
Experience with laryngeal tumors in children is very scarce and has been limited to case reports and institutional re- views[11],[12].
Children may present with hoarseness, dysphagia, stridor, or upper airway obstruction. The severity of the clinical picture de- pends on the degree of airway obstruction caused by the tumor. Diagnosis is completed with endoscopic evaluation of the air- way, airway biopsy, and other studies including computed axial tomography and magnetic resonance imaging of the neck to assess the extent of the disease. The definitive diagnosis of laryngeal lesions is made by endoscopic findings and biopsy with histopathological examination[13].
The first case of laryngeal cancer (squamous cell carcinoma) in a 3-year-old boy was reported by Rehn in 1868. In 1969, Jones and Gabriel conducted a literature review with 99 cases of laryngeal carcinoma in patients under 20 years.
Subsequently, Gindhart et al in 1980, in a review of the literature, found 38 publications with 54 reported cases of squamous carcinoma of the larynx in children under 15 years of age since 1968. Of the reported cases, 13.4% were in patients between 1-5 years, 23% between 6-10 years, and 66.6% in patients between 11-15 years. It should be noted that there were no cases reported in patients under 1 year of age[14],[15].
Certain subgroups of head and neck malignancies are significantly less common in pediatric patients, in particular sarcomas. Sarcomas account for approximately 1% of all head and neck malignancies.
Soft tissue sarcoma represents a diverse class of malignancies. They can also be divided by histological subtypes such as rhabdomyosarcoma, chondrosarcoma, Ewing’s sarcoma and fibrosarcoma among others; each with a different biological behavior.
The most common histological subtype of childhood sarcoma is rhabdomyosarcoma, which accounts for approximately 4.5% of all pediatric malignancies with an incidence of 4.5/1,000,000. The distribution of the age of presentation is bimodal: the first peak is between the ages of 2 and 6 years and the second peak between the ages of 10 and 18 years. Among them, the incidence of laryngeal sarcoma is less than 0.1%[16],[17].
Advances in cancer treatments over the past decades have substantially improved sarcoma outcomes. Surgery was the only therapy available in the early 20th century associated with very poor outcomes, however this has changed with the advent of multimodal therapy[18].
Levi et al., conducted a systematic review of the literature of cases of laryngeal sarcoma in children from 1980 to 2019. The objective of the study was to evaluate the histological types and the treatment received in patients with laryngeal sarcoma in children up to 16 years of age. A total of 29 articles (37 patients) met the inclusion criteria for the study analysis. The median age identified in the systematic review was 11 years with a range of 0 to 16 years. 79.4% of the patients were men and 20.6% were women. The most common histological sub- type of sarcoma was rhabdomyosarcoma, present in 69.4% of the cases. 19.4% of the patients were diagnosed with synovial sarcoma. The rest of histological types such as chondrosarcoma (2.8%), Ewing’s sarcoma (2.8%), fibrosarcoma (2.8%) and mixed sarcoma (2.8%) were diagnosed in 1 patient each. The different locations of the tumor were: supraglottic (62.1%), glottic (17.2%), and subglottic (20.7%).
Treatment of these tumors was generally defined by size, location, and degree of malignancy. The most common treatment strategy was multimodal (76%) consisting of surgery with chemotherapy and/or irradiation. The patients who received a single treatment modality were distributed as follows: surgery (17.2%), chemotherapy (3.4%), radiotherapy (3.4%). On the other hand, the patients who received bimodal therapy were distributed as follows: chemo and radiotherapy (17.2%), surgery and chemotherapy (10.3%), surgery and radiotherapy (20.7%) and the rest of the patients were treated with surgery, chemotherapy, and radiotherapy (27.6%). Among all patients who underwent initial removal or ablation surgeries, 50% underwent total laryngectomy, while the other 50% had laryn- geal preservation procedures such as partial laryngectomy or endoscopic resection of the laryngeal lesion[19].
“Children are not small adults”. This commonly mentioned phrase could not be more relevant than in the care of our smallest and most fragile patients such as patients of a short age of life. Advances in pediatric anesthesia have contributed to improved outcomes and survival in newborns requiring low to high complexity surgical interventions. The physiology of the newborn is characterized by a high metabolic rate, a decreased cardiopulmonary reserve, and a decrease in kidney function, among others. The immaturity of all organs and systems make these patients especially vulnerable during the perioperative period with a high risk of morbidity and mortality compared to other older pediatric and adult patients.
Recent research on perioperative mortality has considered the interaction of patient comorbidities with the intrinsic risk of the surgical procedure. Nasr et al. identified 5 main comorbid conditions that affect outcomes and increase mortality: patient weight less than 5 kg, ASA III classification or greater, ventilatory support, preoperative sepsis, and inotropic support[20],[21].
It should be noted that our patient had 3 of those risks fac- tors: previous ventilatory support, weight less than 5 kg, and ASA III.
The approach of complex pathology of the airway in neo- nates is a great challenge for anesthesiologists considering the limited literature on the matter, because generally airway procedures are meant for other pathologies such as: laryngomalacia, subglottic cysts, subglottic hemangiomas, laryngeal cleft, subglottic stenosis, laryngeal papillomatosis, tracheomalacia.
The management of our highly complex patient was possible due to the multidisciplinary approach and the preparation prior to the procedure of the otorhinolaryngology, anesthesia, and critical care group[22],[23],[24],[25],[26].
From the anesthetic point of view, the preoperative evaluation, determination of the surgical risk, explanation of the in- formed consent with the risks to the parents and the confirmation of the reservation of blood products were key to the good outcome of the case.
During the intraoperative period, the preparation of the operating room with the devices for airway management, canalization of the necessary vascular accesses, adequate hemodynamic, gasimetric and temperature monitoring, appropriate mechanical ventilation, availability of medications, intravenous fluids and blood products and the availability of critical postoperative care were fundamental for the success in the management of this very vulnerable patient.
The risks associated with the critical points of total laryngectomy surgery reported in adults were considered, such as: cervical incision, performance of the tracheal stoma, hemorrhage secondary to injury to neck and thyroid vessels, thyroid and thymus injury, pneumothorax, pneumediastinum, and pneumo- pericardium risks, endotracheal tube balloon damage, need to change the tracheal tube for another more proximal tube with the imminent risk of loss of the tracheal lumen, obstruction of the tube with blood or secretions, mono bronchial intubation, accidental extubation or kinking of the tube causing difficulties for ventilation[27],[28],[29].
In addition to basic ASA monitoring, invasive blood pressure monitoring was justified because of the need for taking serial gases and continuous blood pressure monitoring. Even though total laryngectomy in an adult patient does not mean a high risk of bleeding, in a neonate with a lower intravascular volume in relation to weight, bleeding leads to a high risk of hemodynamic instability, shock, and electrolyte disturbances. During the intraoperative period, the monitoring and maintenance of temperature was guaranteed, considering the high risk of hypothermia in this age group.
Close monitoring of ventilatory parameters was performed by monitoring mechanical ventilation, arterial gases, and by measuring the difference in PaCO2 and ETCO2 to detect in- creased dead space due to the nature of the procedure and the impact of this on the physiology of the patient, in addition to the risk of recurrent and inadvertent mobilization of the tracheal tube due to surgical manipulation.
Furthermore, the patency of the endotracheal tube was continuously monitored due to the risk of obstruction with blood and/or secretions in a small-caliber tracheal tube. During normal circumstances, the flow through the airway is laminar and follows the Hagen – Poiseuille principle; flow velocity is directly proportional to the pressure gradient and the transverse diameter of the airway. A narrow airway causes turbulent air- flow and a higher-pressure gradient, an aspect of great importance due to the risk of difficult ventilation during the intraoperative period[30].
Careful administration of fluids, especially to avoid overload is imperative for younger children. The prevention of hypoglycemia is essential in this group of patients due to their young age with high metabolic consumption and low glycogen reserve; therefore, a perioperative metabolic flow must be ensured. Due to the few permissible blood losses in neonates, it is usually necessary to perform transfusion of blood products; the risks that this entail, such as hyperkalemia, hypocalcemia, volume overload and dilution of coagulation factors must be considered.
Risk reduction strategies include ordering fresh and washed red blood cells, administering diuretics prophylactically (furosemide) to prevent hypervolemia, intravenous calcium in the presence of hypocalcemia and monitoring for signs of hyperkalemia on the electrocardiogram.
One of the most challenging tasks an anesthesiologist can face is providing safe and quality anesthesia for surgery on a newborn. It requires a deep understanding of the anatomy, physiology, and rapidly changing pathology of the neonate as well as the pharmacokinetics and pharmacodynamics of the ad- ministered drugs. This knowledge must be incorporated into a well-programmed anesthetic care plan, likewise, manual skills and ongoing experience with the unique challenges posed by neonates are essential for optimal clinical outcomes with these highly vulnerable patients.
-
Conclusions
End laryngotracheal surgery in newborns, infants and children poses a great challenge for both the anesthesiologist and the otolaryngologist.
The diameter of the airway, the smaller size of the nervous and vascular structures, the small weight, the immaturity of all organs and systems, especially in neonates, and the great variability of pathological lesions require close collaboration be- tween the anesthesia and surgical team to provide optimal surgical conditions and ensure adequate oxygenation, ventilation and perfusion during the perioperative period and thus obtain a good surgical result vital for the prognosis of the disease.
Due to the infrequency of laryngeal carcinoma in children, there is no consensus on the most relevant therapy.
Treatment of laryngeal carcinoma in pediatric patients presents several difficulties; it is difficult to inform parents and relatives about the disease, treatment decisions, and its late consequences.
It is of great importance to report these cases because the reviews of clinical cases in the literature are not numerous enough to make treatment decisions due to the lack of sufficient information on staging, management, and results. Accurate diagnosis and detection of an early stage of the disease are essential for the treatment of pediatric laryngeal carcinoma.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424. https://doi.org/10.3322/caac.21492 PMID:30207593
2. Olgun Y, Erdag TK, Aydin B, Mutafoglu K, Ozer E, Ikiz AO, et al. Pediatric laryngeal cancer with 5-year follow up: case report. Int J Pediatr Otorhinolaryngol. 2013 Jul;77(7):1215–8. https://doi.org/10.1016/j.ijporl.2013.04.019 PMID:23673162
3. Swain SK, Sahu MC. Laryngeal Carcinoma in a Pediatric Patient – A Case Report. Iran J Otorhinolaryngol. 2019 Jul;31(105):251–5. PMID:31384594
4. Abramowsky CR, Witt WJ. Sarcoma of the larynx in a newborn. Cancer. 1983 May;51(9):1726–30. https://doi.org/10.1002/1097-0142(19830501)51:93.0.CO;2-W PMID:6187433
5. Rigby RG, Holinger PH; RIGBY RG. HOLINGER PH. FIBROSARCOMA OF THE LARYNX IN AN INFANT. Arch Otolaryngol Head Neck Surg. 1943;37(3):425–9. https://doi.org/10.1001/archotol.1943.00670030435010.
6. Pankaj C, Devendra C, Prathamesh P, Manish J, Anil PG. Carcinoma Larynx in Children. Int J Head Neck Surg. 2010;1(1):49–51. https://doi.org/10.5005/jp-journals-10001-1010.
7. McGuirt WF Jr, Little JP. Laryngeal cancer in children and adolescents. Otolaryngol Clin North Am. 1997 Apr;30(2):207–14. https://doi.org/10.1016/S0030-6665(20)30240-1 PMID:9052665
8. Cho HJ, Yoon J, Lee E, Lee YS, Kim SY, Roh JL, et al. The Different Clinical Aspects of Pediatric Primary Airway Tumors in the Larynx, Trachea, and Bronchi. J Korean Med Sci. 2017 Aug;32(8):1304–11. https://doi.org/10.3346/jkms.2017.32.8.1304 PMID:28665067
9. Monnier P. Neoplastic Lesions of the Larynx and Trachea. Pediatric Airway Surgery; 2011. pp. 217–27. https://doi.org/10.1007/978-3-642-13535-4_16.
10. Hoetzenecker K, Schweiger T, Denk-Linnert DM, Klepetko W. Pediatric airway surgery. J Thorac Dis. 2017 Jun;9(6):1663–71. https://doi.org/10.21037/jtd.2017.05.50 PMID:28740684
11. Bindlish V, Papsin BC, Gilbert RW. Pediatric laryngeal cancer: case report and review of literature. J Otolaryngol. 2001 Feb;30(1):55–7. https://doi.org/10.2310/7070.2001.20982 PMID:11770976
12. Laurian N, Sadov R, Strauss M, Kessler E. Laryngeal carcinoma in childhood. Report of a case and review of the literature. Laryngoscope. 1984 May;94(5 Pt 1):684–7. https://doi.org/10.1288/00005537-198405000-00021 PMID:6201689
13. Dombrowski ND, Wolter NE, Irace AL, Robson CD, Perez-Atayde AR, Mack JW, et al. Squamous cell carcinoma of the head and neck in children. Int J Pediatr Otorhinolaryngol. 2019 Feb;117:131–7. https://doi.org/10.1016/j.ijporl.2018.11.019 PMID:30579067
14. Gindhart TD, Johnston WH, Chism SE, Dedo HH. Carcinoma of the larynx in childhood. Cancer. 1980 Oct;46(7):1683–7. https://doi.org/10.1002/1097-0142(19801001)46:73.0.CO;2-2 PMID:7417962
15. Ferlito A, Rinaldo A, Marioni G. Laryngeal malignant neoplasms in children and adolescents. Int J Pediatr Otorhinolaryngol. 1999 Jun;49(1):1–14. https://doi.org/10.1016/S0165-5876(99)00038-5 PMID:10428400
16. Hu J, Lu D, Ren J, Wen Q, Zhou J, Gan W, et al. Adult laryngeal Embryonal Rhabdomyosarcoma: a case report and literature review. BMC Surg. 2020 Jul;20(1):173. https://doi.org/10.1186/s12893-020-00831-7 PMID:32736545
17. Moretti G, Guimarães R, Oliveira KM, Sanjar F, Voegels RL. Rhabdomyosarcoma of the head and neck: 24 cases and literature review. Rev Bras Otorrinolaringol (Engl Ed). 2010;76(4):533–7. https://doi.org/10.1590/S1808-86942010000400020 PMID:20835543
18. Forsyth AM, Camilon PR, Tracy L, Levi JR. Pediatric laryngeal tumors and demographics, management, and survival in laryngeal squamous cell carcinoma. Int J Pediatr Otorhinolaryngol. 2021 Jan;140:110507. https://doi.org/10.1016/j.ijporl.2020.110507 PMID:33279850
19. Mur TA, Pellegrini WR, Jaleel Z, Edwards HA, Levi JR. Pediatric laryngeal sarcoma: systematic review and pooled analysis. Int J Pediatr Otorhinolaryngol. 2020 Dec;139:110471. https://doi.org/10.1016/j.ijporl.2020.110471 PMID:33120103
20. Nasr VG, Staffa SJ, Zurakowski D, DiNardo JA, Faraoni D. Pediatric Risk Stratification Is Improved by Integrating Both Patient Comorbidities and Intrinsic Surgical Risk. Anesthesiology. 2019 Jun;130(6):971–80. https://doi.org/10.1097/ALN.0000000000002659 PMID:30946056
21. Kuan CC, Shaw SJ. Anesthesia for Major Surgery in the Neonate. Anesthesiol Clin. 2020 Mar;38(1):1–18. https://doi.org/10.1016/j.anclin.2019.10.001 PMID:32008645
22. Gupta P, Tobias JD, Goyal S, Kuperstock JE, Hashmi SF, Shin J, et al. Perioperative care following complex laryngotracheal reconstruction in infants and children. Saudi J Anaesth. 2010 Sep;4(3):186–96. https://doi.org/10.4103/1658-354X.71577 PMID:21189858
23. Bradley J, Lee GS, Peyton J. Anesthesia for shared airway surgery in children. Paediatr Anaesth. 2020 Mar;30(3):288–95. https://doi.org/10.1111/pan.13815 PMID:31898366
24. Charters P, Ahmad I, Patel A, Russell S. Anaesthesia for head and neck surgery: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016 May;130 S2:S23–7. https://doi.org/10.1017/S0022215116000384 PMID:27841108
25. Collins CE. Anesthesia for pediatric airway surgery: recommendations and review from a pediatric referral center. Anesthesiol Clin. 2010 Sep;28(3):505–17. https://doi.org/10.1016/j.anclin.2010.07.008 PMID:20850081
26. Flory S, Appadurai IR. Special Considerations in Anesthesia for Laryngeal Cancer Surgery. Int J Otorhinolaryngol Clin. 2010;2(3):185–90. https://doi.org/10.5005/jp-journals-10003-1034.
27. Smeltz AM, Bhatia M, Arora H, Long J, Kumar PA. Anesthesia for Resection and Reconstruction of the Trachea and Carina. J Cardiothorac Vasc Anesth. 2020 Jul;34(7):1902–13. https://doi.org/10.1053/j.jvca.2019.10.004 PMID:31761653
28. Nolan M, Beebe D. Anesthesia for reconstructive airway surgery in pediatrics. In: Abdelmalak B, Doyle J, editors. Anesthesia for Otolaryngologic Surgery. 2012. pp. 337–45. https://doi.org/10.1017/CBO9781139088312.036.
29. Hsu J, Tan M. Anesthesia Considerations in Laryngeal Surgery. Int Anesthesiol Clin. 2017;55(1):11–32. https://doi.org/10.1097/AIA.0000000000000126 PMID:27930417
30. Vimmi Oshan FR, Robert WM. Walker, DCH FRCA, Anaesthesia for complex airway surgery in children. Contin Educ Anaesth Crit Care Pain. 2013 Apr;13(2):47–51. https://doi.org/10.1093/bjaceaccp/mks058.

 ORCID
ORCID